The Moderna N-Antibodies Paper
Finally, proof: Early breakthrough infections do not always generate antibodies against the Nucleocapsid protein. Well... Duh.
Moderna-vaccinated trial participants who went on to experience “breakthrough” infections in the first 4 months did not generate N-antibodies, in a suspiciously “Substack-bait-y” study.
Except when they did.
This Again
A recap, with apologies to longer-time readers.
In October, the UK Health Security Agency dropped the following comment into their weekly report, in the portion regarding blood donor antibody levels against either the Spike or Nucleocapsid protein of SARS-CoV-2:
recent observations from UK Health Security Agency (UKHSA) surveillance data [show] that N antibody levels appear to be lower in individuals who acquire infection following 2 doses of vaccination.
And in October, I said the following of the same comment.1
“Recent observations” does not mean observations of recent outcomes. Plenty of “recently” published studies have been time capsules of post-Covid-vaccination infections from the spring [of 2021]. […] Early “breakthrough” infections are less symptomatic, and more prone to false positives; post 4-month “breakthrough” infections are more likely to be the real deal.
And in February, this year, I reprised the subject, and said I am going to be right about this.2 I further noted that N-antibodies were on the rise among donors.
And two months later, I reprised the subject again, and said I am going to be right about this.3 And N-antibodies were on the rise even more. The state of blood-donor samples then:

What “being right” meant, was that the UKHSA October comment about breakthrough infections was not, in fact, a correct explanation for why donor samples were low in N-antibodies in October. Instead, (primarily Covid-vaccinated) Brits were just less likely to donate blood after breakthrough infection, so that the donor sample pool continued to predominately (but less and less predominately) reflect a bias for non-infected, Covid-vaccinated. Simple as that (and, also what was suggested in the first two parts of the UKHSA sentence).
So that’s the recap.
Hurry Up and Wait
And so what was released two weeks after my last revisit to the UKHSA comment on “recent” observations, and half an entire year after my initial remarks?
A time capsule of post-Covid-vaccination infections from even before the spring.4
These results pertain to Moderna Covid vaccine trial participants up to the end of March, 2021 (at which point the trial effectively self-destruct-button’ed via unblinding); which was 4-5 months or so past the date of 2nd injection for most participants, placebo or not.
Nasopharyngeal swabs for SARS-CoV-2 PCR testing were taken from all participants on Day 1 and Day 29 (vaccination days), and during symptom-prompted illness visits. Serum samples from Days 1, 29, 57, and the Participant Decision Visit (PDV, when participants were informed of treatment assignment, median day 149) were tested for anti-N Abs.
The authors looked at the roughly 4-months-from-2nd-dose serum samples for those participants who wound up symptomatic and PCR positive after day 43, to see if any N-antibodies were inside. These “observations” are thus directly comparable to “observations” from real-world breakthrough infections in the first four months after Covid-vaccination.
As everyone knows, spring, 2021 was most breakthrough infections happened. Not afterward, when infection efficacy went to zero and the entire world lost its mind!5 No, in the spring, when no one was talking about the issue at all. So it’s natural that we should be worried about N antibodies in this group of Covid-vaccine recipients (the one that almost no-one in the world is in), as opposed to those who were infected later (the one that loads and loads of Covid-vaccinated are in).
Besides, we can’t look at that N-antibody response in individuals belonging to that other, bigger group. There just aren’t any studies. (There are.6) We have to extrapolate from the data on individuals in the smaller, and now historically obsolete group. Sorry!
So, what do they find? Excluding infections before the normal, 14-day “fully” vaccinated cut-off (i.e., infections before (244 in placebo and 260 in treatment) or during (23 and 31) enrollment, or between enrollment and the cutoff (27 and 12)7):
Of note, already: There sure weren’t many infections in the treatment group. Thus, the infections that did happen were true “breakthrough” infections - they occurred when infection efficacy was still in place (whereas later infections aren’t really “breaking” through anything). However, this protection appears to be waning, as there are more infections in the treatment group in the 53 days before individual unblinding dates (“PDV”) than the ~100 days before then. No surprise, really. But this could also merely reflect background case rates, since the placebo group appears to show the same acceleration. Additionally, some of these will likely be false positives in both groups.
Why does the “late early” Moderna-vaxxed group have an even lower N-antibody rate than the truly, truly early group? I would want to see just how close to day -5 these were. Seroconversion, especially for IgG, occurs on a delay. In fact it is surprising that the placebo group does not show a similar effect, given that seroconversion is delayed in the placebo group for pre-cutoff infections.8
Stop the Post, I Want to Get Off
Let’s say, here, that further comparison of these 52 breakthrough infections with the results for the placebo group refutes my original claim that early breakthrough infections are simply less likely to be the “real deal,” or that this less-likeliness appears to account for the difference.
So what? It would still be the case that these are early breakthrough infections. Why are we even looking at these?
The authors innocently claim that their motivation for trawling back through the early trial results is to answer the questions, “how vaccine induced immunity may impact seroconversion to non-Spike proteins, and whether such immunoassays have similar performance in determining previous SARS-CoV-2 infection in regions of the world with high vaccination coverage.”
What other important questions should we look to the early Moderna trial results to decipher?
Infection efficacy (high), including asymptomatic (not even measured)?
Severe outcome efficacy (high)?
This is an honest question. Both of those two aspects of Covid vaccine immune response were intentionally distorted by the trial’s early cut-off (unblinding). Why wouldn’t N-antibody seroconversion also be distorted by same, even if not in the exact manner I speculated in October (due to milder or asymptomatic infections, or false positives)?
And why should we stop there? There are still other findings from studies of early real-world breakthrough infections that we can add to our knowledge base:
Viral load of Breakthrough infections (lower)
Time to PCR-negativity of Breakthrough infections (shorter)
Is anyone who would use the Moderna trial N-antibody results to divine some truth about the world, also proposing we suddenly take up a vehement belief in 93% infection efficacy, severe efficacy, lower viral load, and shorter duration of viral shedding? They might as well rename their substacks Your Other Local Epidemiologist in that case.
How Do You Figure
Helpful as it might be, a granular breakdown of the days of infection for each group is not provided. So, beyond Table 1, the reader is hostage to however the authors decide to present the data. Which leads us to Figure 2.
In A (which definitely hasn’t been cropped out by anyone else reporting on this study), the authors show viral load for seronegative and seropositive “Covid-19 infections” in the placebo and vaccine groups. This analysis uses the same definition as Table 1, above, but removes any reference to the time of infection. All that is known is viral load (as inferred by PCR cycle count) for the SARS-CoV-2-positive swab taken when at least two symptoms were reported. This is how I like to look at the data. It is just a bunch of dots. I can sit with them, over the course of how ever many minutes I like, and just think about what I am seeing.
In B, it’s the same data, but now the authors are shoving a conclusion down the reader’s throat. It is what they saw when they looked at A.
What the authors saw (B):
The placebo group is much more likely to be N-antibody positive at the end of the trial when their during-trial infections were accompanied by a low viral load, than the vaccine group when their during-trial infections were accompanied by a low viral load.
Via statistical distortion which is completely inappropriate on such a small sample size, the Covid-vaccinated can be made to “show” that seropositivity rates are still lagging even at “5” viral load. This is obvious nonsense. Since all breakthrough infections between “5” and “7.5” are seropositive, the probability of being seropositive at all these values is 100%! The “curve” in B is just a stupid, mathematically-fabricated lie.
What I see (A):
Simply by crafting the design of B according to their conclusion, the authors are able to obscure everything else that can be deciphered in A. That does, however, leave the observation that B exaggerates into oblivion as apparently valid: Early breakthrough infections with low viral loads do not appear to generate N antibodies as much as natural infections with low viral loads.
But so what? It still means that early breakthrough infections appear not to lead to N-antibodies because of low viral loads. And, studies of breakthrough infections from after spring revealed that the Covid vaccinated cease to be more likely to have lower viral loads after infection efficacy drops.9 So this would essentially affirm what I said in October.
At best, you could say that “When the Covid vaccine response is enough to keep infections mild, non-spike antibodies seem to appear less often; whereas for natural infections that are kept mild by innate immunity, non-spike antibodies appear more often.” But even to say this is to use early breakthrough infections as a window into what most post-Covid vaccine infections are like for only this one thing, when we know it doesn’t serve well as a window for any other aspect.
It further risks the possibility that many of the Covid-vaccinated seronegative were sampled too early for seroconversion (since, again, 28 of 52 breakthrough infections were dated closer to the serum sample).
It further just pretends that the Covid vaccinated aren’t outperforming the placebo group “when it counts,” in the high viral load infections.
It further just pretends that the authors did not fail to include the age of breakthrough infections (by N status10), whether for early-early vs late-early, or low viral load vs high. That’s not suspicious. It’s very typical not to report age, which affects immune response, when analyzing immune responses in trial data that include age, which affects immune response.
It further ignores that a handful of low viral load infections in both groups are likely false positives (as I said in October): At least some participants in either group likely experienced qualifying symptoms (such as “cough,” or “headache”11), and were false-PCR-positives during visit. How many?
Among the ~14.5 thousand participants in each group over the course of the study?
5?
25?
The former wouldn’t matter much; the latter would account for almost all of the observed N-seronegative breakthrough “infections.”12
Counter-Ntel
Both this paper and the “Macaque” paper13 have suspicious author affiliations. Obviously, to point these affiliations out only makes my analysis look weaker; but I also find these affiliations intrinsically notable. They are one piece of a larger puzzle, part of which includes the bizarre timing of the resurrection of OAS from the dustbin of scientific history over the last few decades to begin with; another part of which includes the fact that the insinuation of the OAS myth into Covid vaccine skeptical substack discourse is largely, if not entirely, the work of a few anonymous authors.
For today’s study - which can only be taken as connected to OAS if one first presumes that the N-antibody controversy was ever related to the question in the first place - take Lindsey R. Baden, who occupies the “executive-producer”-esque final spot on the author list:

L.R.B. declares support (in the form of grants paid to his institution) from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) for the conduct of this study as well as grants (paid to his institution) within the last 36 months from NIH/NIAID, Gates Foundation, the Ragon Institute, and Wellcome Trust, outside the submitted work. L.R.B. is involved in HIV, other pathogens, and COVID vaccine clinical trials conducted in collaboration with the NIH, HIV Vaccine Trials Network (HVTN), COVID Vaccine Prevention Network (CoVPN), International AIDS Vaccine Initiative (IAVI), Crucell/Janssen, Moderna, Military HIV Research Program (MHRP), Gates Foundation, and the Ragon Institute.
Do any of these reviled institutions14 potentially want to promote a belief in the myth of OAS (or, N-antibody seronegativity confused for OAS)? Is that why the Moderna trial results have suddenly been revived from the grave to unleash this almost prankish, facetiously backwards study design upon the world?
What would be the reason for their wanting this?
Could it be, because OAS is a farcical biological myth, developed in an era when antibodies were thought to be the be-all-and-end-all of immunity and before we even understood what the innate immune system is, that now makes anyone who talks about it look ridiculous?
Could it be, because this entire discussion detracts from the direct injuries and deaths caused by the Covid vaccines?
Elsewhere, in the OAS saga:
“Even-Steven.” (OAS is “proven” via generation of a more balanced immune response against Alpha or Delta after breakthrough infection.)
“Macaque Me an Offer I Can’t Refuse.” (OAS is “proven” via animal models where mRNA-vaccinated hamsters and macaques do better than unvaccinated controls vs Omicron.)
“Original Antigroundhogic Sin.” (OAS is “proven” via breakthrough blood samples taken too early for seroconversion, and an overhyped UKHSA comment.)
“Funeral for a Fact,” footnote 3. (OAS is “proven” because the boosters restore infection efficacy vs. Delta during the Omicron wave.)
“Darmok and the Spike Protein at Tanagra.” (OAS is “proven” when high pre-existing antibodies against coronaviruses… don’t correlate to severe outcomes in infection with SARS-CoV-2 in any way.)
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
See “(Not) Coming Up!”
See “Original Antigroundhogic Sin.” Not that footnote 6 might also include Servellita, et al., the study reviewed in “Antigroundhogic.” However, as discussed in that post, Omicron breakthrough blood samples were taken too early for seroconversion.
See “The Hot Spot.”
Follmann, D. et al. “Anti-nucleocapsid antibodies following SARS-CoV-2 infection in the blinded phase of the mRNA-1273 Covid-19 vaccine efficacy clinical trial.” medrxiv.org
For a trip down vax-failure-freakout memory lane, see “Near t' Rock Bottom out on Route 23.”
See:
Minervina, A. et al. “SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells.” Nature Immunology (2022)
(Hat tip to reader “OhioPatriot,” since this paper had been in preprint for months and yet totally under my radar, so I might never have taken a look at it.)
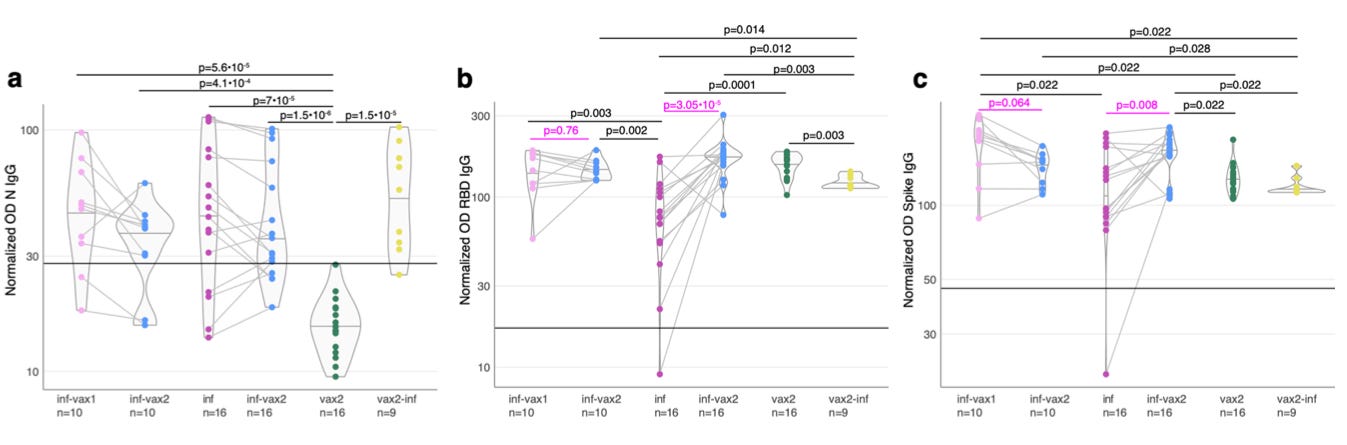
In which, N antibody levels are higher among Covid-vaccinated + infected (n=9) than every other cohort, including natural-infected.
And, in T Cell land:
Given the potent induction and expansion of spike-specific responses by vaccination, even in individuals who were previously infected, we predicted that infection of previously vaccinated individuals (breakthrough, vax2-inf) would maintain a spike-specific bias. Surprisingly, [it shouldn’t be surprising!] we observed a large non-spike-specific T cell response in the majority of the breakthrough 6/7 (vax2-inf) cohort (Fig. 2h), indicating that a robust primary response to non-spike SARS-CoV-2 antigens during the breakthrough infection is not impaired by the presence of spike-specific immune memory elicited by vaccination [there is no reason it would have been!]. The ratio between spike-specific and non-spike-specific T cells in breakthrough cases (vax2-inf) was no different from that of donors who were only infected (inf; P = 0.97, Mann–Whitney U test), indicating that the T cell response to the non-spike antigens is of comparable magnitude among those who were only infected and among those who experienced breakthrough infection after vaccination (vax2-inf).
See also the PHE N-antibody study reviewed in “The Hot Spot,” footnote 6, and also discussed here in footnote 8.
For breakthrough immune response studies not specific to the N protein, see “Even Steven” (number of breakthrough infected=12). Also, “BioNTech Omicron Breakthrough Study!” (n=16). Also, “The Austria Omicron Study” (n=27). Also, footnote 2.
Fewer PCR positives between baseline and Day 29 in the mRNA-1273 group vs. placebo?!
Surely other, anonymously-helmed Covid-vaccine-skeptical substacks, pillars of integrity that they are, are rushing to report this startling refutation of the mythical “Worry Window” upon which they have also premised the entire super-structures of their theories about severe non-efficacy and OAS (sarcasm). Note that Day 30 to 42 are missing for both groups in this analysis, based on the 14 days post dose-2 definition for the “illness visit” sets.
Note that since I do not believe in the “Worry Window” or the “benefit” of a 2nd injection, I would have chosen to include these 27 and 12 Day 29 PCR positives (which are universally N seronegative, but convert afterward) in the main, “illness visit” analysis, even though a few will be false positives in both groups.
As shown in footnote 7.
For the 30 placebo-group “new PCR+ on Day 29” to be only ~60% N-seropositive on Day 57 is reasonable (as is for the vaccine group to be less so, given that the status of being “vaccinated” (having high anti-spike antibodies that temporarily reduce likelihood of infection) takes effect shortly after Dose 1, not Dose 2).
So if ~40% placebo group N-seronegativity is reasonable ~28 days after infection, why aren’t ~20% of the placebo group N-seronegative in the “-5-53 days PDV [serum sample day] PCR+” group?
Well, maybe because the sky-high seropositivity rates in Table 1 are some sort of “error.” But also, maybe because ~30 of that ~40% were false positives to begin with, given that Day 29 PCR testing was issued regardless of symptoms (as noted). However, an assumption of equal absolute counts of Day 29 false-positivity suggests that 30 * .3 is too high (since a maximum of 6 in the treatment group could be false-PCR+ on Day 29), so… that doesn’t help much.
But the final “maybe,” or more like a probably, is that the days to PDV grouping in Table 1 is deceptive.
The authors imply that their decision to “stratify” results strictly by side-of-the-mean (half of infections before this relative-to-PDV-cutoff, half after) were to demonstrate similar seropositivity (apparently meaning, in the placebo group). So they are acknowledging that timing could be important, but taking the most narrow means of testing against that hazard possible. At the least it’s a very grade-your-own-homework-y approach; at worst it’s hiding a disparity in timing between the placebo and vaccine groups.
For the placebo group, the “5 to 53” days before the end of March would essentially describe “February and March, 2021.” These infections might skew more to February, just after the winter wave, accounting for the otherwise weirdly high N-seropositivity (so almost all in this group were infected long enough before PDV to generate antibodies; also note that 54-150 day infections might skew to January, explaining why the longer period does not have more infections, because it wasn’t really longer in “wave time”). For the vaccine group, infections during February and March would skew to March, as infection efficacy began to wane, or as the Canada-East Coast “mini spring 2021 wave” began to take off (which itself might have been driven by vaccine failure).
By not showing the actual plot of infection dates, the authors could be creating an intentionally deceptive comparison between a predominately two-month-post-PCR+ placebo set and predominately one-month-post-PCR+ vaccine set.
Another hint that this might be the case, and that something is “off” with the low N-antibody rate in the Covid vaccine group in this paper, comes from a little-noticed Public Health England study which may actually have been the source of the “recent observations” mentioned by the UKHSA (reviewed in “The Hot Spot,” footnote 6):
Whitaker, H. et al. “Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off.” medrxiv.org.
In this, not only are samples after “possible late breakthrough, Delta” infections among the double-dosed almost universally (42 out of 44) N-seropositive, but samples after “probable early breakthrough, Alpha” infections are 78%. This is a lot more than the 32.1% rate for the -5-53-day group reported by the Moderna paper. Why the difference? Because Whitaker, et al. made sure to use sample-intervals that allowed time for seroconversion.
So it seems that a skew in sample timing (i.e., time from PCR-positive to individual participant unblinding) probably account for the aberrant results in some form (with possible high false positives in the -54-150 Covid vaccine infections group).
But “probably” doesn’t rule out the other “maybes.” Note that in the higher column of Table 3, Day 57 N-seropositivity rates are only 86.9% among the 61 placebo participants who were (newly) N-seropositive at Day 29. Since there is no PCR-false-positive hazard here, this effectively means that due to “random N-positivity loss,” a sample of 324 recently infected placebo participants shouldn’t exceed ~87% positive; however, that number may not be accurate thanks to the smaller sample.
You be the judge. Personally, I’m totally sure there’s no reason the authors would be motivated to “err” on the side of an over-high N-positive placebo rate in Table 1.
See eTable 2 for ages. Covid-vaccinated “breakthrough” infections are a “tiny bit” (official math) older than placebo group, but that doesn’t mean anything without knowing age by N-antibody status.
(Follmann, D. et al.)
Covid-19 cases are defined as baseline SARS-CoV-2 negative participants in the per protocol population, who had at least two of the following symptoms: fever (temperature ≥38°C), chills, myalgia, headache, sore throat, or new olfactory or taste disorder, or as occurring in those who had at least one respiratory sign or symptom (including cough, shortness of breath, or clinical or radiographic evidence of pneumonia), and at least one NP swab, nasal swab, or saliva sample (or respiratory sample, if the participant was hospitalized) that was positive for SARS-CoV-2 by RT-PCR
See “Macaque to the Future.”
Though, whatever it is, I like the sound of “COVID Vaccine Prevention Network.”


















This is exactly what I thought when seeing those claims flying around so nice to read a supporting analysis from someone who understands the biology. I think that what I call the canonical counter-narrative has become as much a religion for many people as Covidism. The problem with that is that it is a major distraction from the issues we should be concerned with (so much so, that it wouldn't surprise me if major elements of this counter-narrative were in fact deliberately seeded from the same Covidist sources; even if I prefer to the extent possible to see these things as emergent social phenomena)
> At best, you could say that “When the Covid vaccine response is enough to keep infections mild, non-spike antibodies seem to appear less often; whereas for natural infections that are kept mild by innate immunity, non-spike antibodies appear more often.” But even to say this is to use early breakthrough infections as a window into what most post-Covid vaccine infections are like for only this one thing, when we know it doesn’t serve well as a window for any other aspect.
So for natural unvaxed mild infections, people make N antibodies. But for vaxed mild infections the vaxed do not make them? That IS OAS.
This is the beauty of natural immunity: have a mild illness, get long term immunity. The unvaxed do not need to have a severe illness to become immune. Thank You to wisdom of God who created us with immune systems (or Nature, for atheists). F U to the vaccine which prevents true immunity from forming.