The Hep B vax HIV origin theory, pt. 3
On the likely safety of the wild, crazy, blood-based Hepatitis B vaccine.
Onto safety…
In Pt.s 1 and 1.5 of this historical safari, a simple case was summarized and expounded for why it is implausible that HIV spread to humans via weird experiments involving co-circulation of chimpanzee blood with human patients as so provocatively proposed.
Pt. 2 began a focus on the question of whether the Hilleman vaccine trials amplified early spread of HIV, providing an overview of the development and trial process and concluding that evidence regarding HIV infection rates of trial participants is not particularly conclusive in either direction.
As previously summarized, the series will now review the following additional evidence: The process for producing the FHHBV makes it quite implausible that it was contaminated with any viruses even before extra sterilization; the FHHBV was trialed in dialysis patients and used in infants in New Zealand and elsewhere without apparent HIV outbreaks, which seems as good a proof as any; and in perspective the FHHBV even if contaminated could hardly have done more to disseminate the virus than events in Haiti in the 1970s, the practice of blood transfusion generally, and the known risk factors associated with outbreaks after 1978.
On the safety and process of the FHHBV: context
To remind the reader, the genetic record of HIV-1M leaves us uninterested in the question of whether a chimpanzee virus of any type somehow contaminated the “First Hilleman Hepatitis B Vaccine” (FHHBV), because HIV-1M came from decades of human circulation in the Congo region — 1M subgroup B, which exploded in the US, being in fact a rare local offshoot of the 1M family (in that region) at the time. As such we are merely interested in whether the FHHBV, because it was based on the blood (plasma) of chronically Hepatitis-B-infected American donors, a group at high risk for sexually transmitted diseases, was contaminated with human-donor-derived HIV.
The process for producing the FHHBV makes this seem implausible.
The context, it can briefly be noted, also renders this hypothetical a bit academic. If we are worried that the Hepatitis B vaccine was contaminated with HIV because donors were high risk, then said donors were also at risk, already, of spreading the virus into the gay community at large sexually — and the network dynamics of rapid multi-partner-per-month sex ensure that the virus would afterward explode in the community exponentially. Could the FHHBV be halted, there isn’t anything that would seemingly “stop history” so long as a hypothetically HIV-infected donor for the FHHBV is simultaneously sexually active. And, as previously discussed, the timing of the licensing of the vaccine — June, 1981 — means that the writing was already on the wall by the time the far more limited trials concluded. Therefore only some type of miraculous tragedy — i.e., there was an HIV-infected American who donated for the FHHBV trial lots who was also at that time the only HIV-infected American — would allow the FHHBV to really “amplify” the onset of the epidemic. Nonetheless, as previously examined, we can’t really ignore the awfully coincidental timing outright.

SV40
As a useful note on context, it is still somewhat well-remembered that one of the polio vaccines turned out to have been contaminated with a “passenger” virus, the monkey virus SV40, though no harm seems to have resulted from this accidental decades-long “gain of function” experiment despite an alarming tendency for the virus to be found in diseased human tissues.1 Depending on the process used for culturing the Salk vaccine, the amount of SV40 contamination in a given batch could be so tremendous that the .1% of virus which survived formalin inactivation still presented a serious chance of infecting recipients up to 1963.2 Likewise, HIV-infected blood (or plasma) can contain thousands to millions of copies of HIV RNA per mL, trending higher in more advanced (symptomatic) disease states. In short, unexpected concentrations of unexpected contaminants on the inputs of the product mean that sterilization or inactivation alone shouldn’t be considered satisfactory for the FHHBV, in absence of any HIV-specific testing.
Insert: Was Hilleman stupid?
In retrospect, the idea of making a vaccine from Hepatitis B-infected gay, male American blood from 1976 to 19813 is obviously crazy. Even at the time, one wonders at the thinking — that it turned out that this population was soon to become the breeding ground for an “unforeseen” blood-transmissible viral infection doesn’t in fact seem very unforeseeable at all.
Ignorance of the SV40 fiasco could not be at play in Hilleman’s thinking — he was instrumental in the early detection and characterization of this and other simian viral contaminants of cell cultures for vaccines.4
Nor could general stupidity or incompetence be at play — Maurice Hilleman was in 1976 already one of the most successful and prolific developers and improvers of vaccines who had ever lived (and remains so today). He was prone to getting things right on the first try — in 1957, he developed the H2N2 (“Asian flu”) vaccine which proved a success when the virus hit American shores months later5, and the improved “breed” of attenuated measles which he produced in 1968 remains the strain in use in the US today.6
Nor did his dominance in American vaccinology depend on the advantage of “simpler times,” when immunological problems were solved without any understanding of the underlying process. In the last two decades of his life, up to his death in 2005, he retained a seemingly unsurpassable familiarity with the state of knowledge on viruses and immunology — thus he was able to write multiple in-depth comparisons of measles, HIV, and Hepatitis B without sharing the byline.7 Perhaps no man is more responsible for the advancement of vaccines in the late 20th Century and the bloating of the American vaccine schedule (whatever one may think of the same8).
The peculiarity of his work on a blood-based Hepatitis B vaccine — at a time when Hepatitis B infection, itself, was proving an unintended consequence of many new medical procedures regarding injection and transfusion — therefore might simply reflect high confidence in his methods of purification and sterilization. This, we will next see, was not unfounded. An additional justification for this strategy is that the alternative to vaccination, in high-risk groups, was infected-blood-derived “immune globulin” administered therapeutically after birth (for newborns of infected mothers) or other exposure. Creating a vaccine from blood thus did not in of itself obviously introduce a novel danger for anyone at risk of Hepatitis B.
Nonetheless, it remains an irony that the man partly responsible for discovering SV40 would not anticipate discovery of a new potential vaccine contaminant which he could not have screened for in advance.
On the safety and process of the FHHBV: details
We have preemptively dismissed sterilization without HIV-specific screening, but what in fact makes it implausible that the FHHBV was contaminated with HIV (even if donors were infected) is that purification of the “antigen” began with stratifying samples by size via density gradient centrifugation.
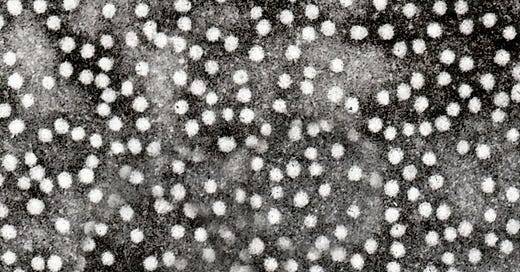
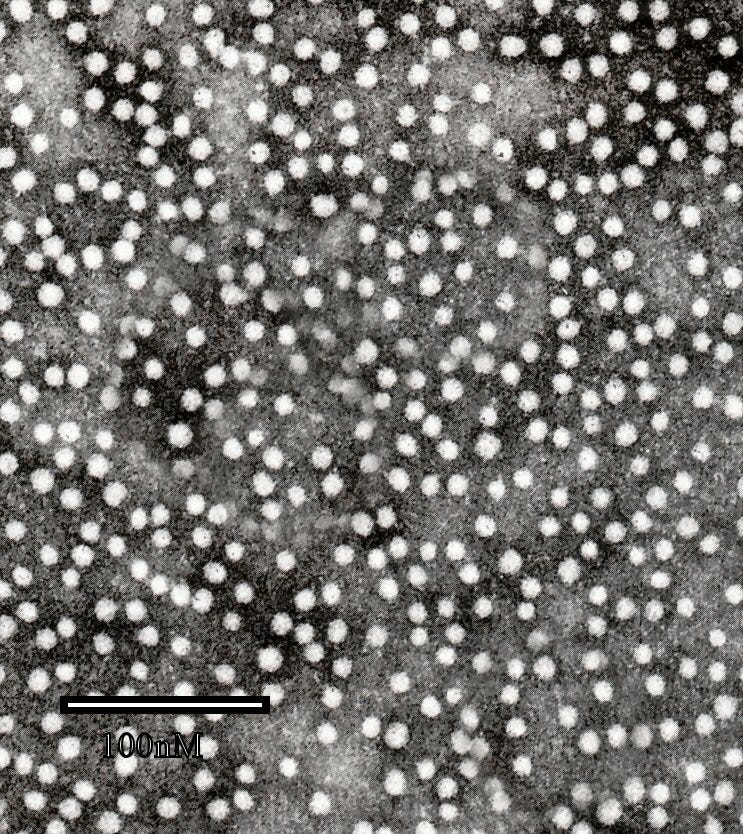
The previously-described “empty” HBsAg used for the FHHBV is a particle 21 - 22nm in diameter. Thus any “whole” Hepatitis B virus, if it was also in donor plasma, would not be in the same band after centrifugation, because it is a larger 42nm. HIV virus would be even further from the HBsAg, at a whopping 100nm — the size of the white bar on the previously shown image.
At 100nm, HIV is not going to find its way into or even near the same band that contains HBsAg and is selected for further purification. This is remarked upon by Hilleman et al. in early descriptions of the vaccine:9
There are, however, other determinants of safety. The density gradient centrifugation used in the purification process separates the small, non-infectious 22-mμ HBzAg particles used to prepare the vaccine from the 42-mμ infectious Dane particles.
Regarding subsequent sterilization, about which we care not-as-much, it was extensive. Quality control steps were additionally taken to ensure that the vaccine was not contaminated with either Hepatitis A10 or B11. The trials themselves best prove the success of these efforts, as both low-risk and high-risk vaccinated human participants were closely tracked for signs of Hepatitis B infection — despite sampling infected donors, the product was free of live whole virus.
The hypothetical potential that donor HIV virons outnumbered Hepatitis B (these were chronic, but otherwise apparently overtly healthy infections) remains a confounder — but this is partially answered by a test in which 100X doses of the FHHBV were given to chimpanzees without any sign of Hepatitis B (or outright death, if we are imagining HIV to somehow be in these test lots at high concentration). And, again, HIV regardless of concentration would be even further away than Hepatitis B after centrifuging; it would have been purified out.
True, it remains the case that absent the application of any-HIV specific assay during testing or manufacture quality control, there is no way to rule out a mishap of some vague form in any given lot of the FHHBV that was produced, whether used in the trials or commercially. But the purity and quality of the vaccine would have suffered in kind; the interest in quality was aligned with the interest in the total removal of unknown agents of the wrong size. Hence, Hilleman et al. write that the absence of “other human blood proteins” and “blood group substances” was demonstrated by conventional tests of the time.
This satisfactorily renders the idea of HIV contamination implausible.
“Canaries” in the vaccine-mine: Dialysis patients and babies at home and abroad
To great extent, the “Hepatitis B vaccine HIV theory” depends on the notion that in the West, no one besides gay men and drug addicts was interested in or targeted for the blood-based FHHBV in trials or commercially. The trials have been reviewed, but I have not performed an exhaustive investigation of who actually received the commercial vaccine — it suffices to simply to point out a few readily available exceptions.
The FHHBV was promptly tested in dialysis patients — since Hepatitis B is transmissible via sharing of blood, the contamination of newly-invented dialysis machines had proven a problem for the centers seeking to milk this new class of human cash cows. The American trial, headed by Wolf Szmuness, encompassed “nearly 900 patients,” but may have only published a limited sample of results.12 It is not particularly plausible that HIV contamination would have gone unnoticed in this context — but at the same time, if we are probing spooky medical experimentation conspiracy theories then we might prefer to look for examples outside of the US.
At least one center in France and one in Germany sought out a contemporary vaccine developed by Institut Pasteur;13 but the University of Milan in Italy used the Merck FHHBV.14 In all, 31 adult patients, 6 child patients, and 48 staff were given the vaccine. This is one example of a foreign employment of the FHHBV which likely would have resulted in great scandal and attention, were the thing contaminated with HIV — after all, there were paychecks at stake. There were likely other such trials, but the trial in Milan suffices.
The other “canary” in the FHHBV coal mine which seems not to have produced any notice of HIV contamination are high-risk newborns. In the US, babies of mothers from high-risk lands were given the vaccine if their mothers were found to be chronically infected — the example shown in Pt. 2 mentioned 25 Asian immigrant mothers in San Francisco as of September, 1982. This program also took place in other cities with significant populations of recent East-Asian immigrants (Los Angeles and New York).
Finally, we turn to (Alaska, Taiwan, and) New Zealand. The extensive employment of the FHHBV vaccine in children in these regions is recorded in a CDC report on the next-generation, yeast-derived subunit vaccine from 1991.15 To quote:
Until recently, large-scale hepatitis B vaccination programs for infants (e.g., Taiwan, Alaska, and New Zealand) have primarily used plasma-derived hepatitis B vaccine. [As of 1991,] No association has been found between vaccination and the occurrence of severe adverse events, including seizures and GBS (55, B. McMahon and A. Milne, unpublished data). However, systematic surveillance for adverse reactions has been limited in these populations, and only a small number of children have received recombinant vaccine
Again, to minimize our scope, we may simply inspect the example of New Zealand. It would seem that the CDC wording elides the detail that the FHHBV was in fact tested in children between 2-12 years old in this country, as well as infants. In sum, at least 6,000 children in heavily indigenous (Māori and mixed-race) boroughs were given diluted doses of the FHHBV in New Zealand.16
It is really not plausible that a FHHBV-induced HIV child holocaust in New Zealand would have gone unnoticed afterward. Therefore, since the FHHBV was given to children in these communities in such enormous quantities without later disaster, it is safe to conclude (regardless of minimal dilution) that it was not a vector for HIV.
Likewise, the decisive factor which explains why other target demographics for the FHHBV — gay men, and drug users — did wind up with explosive outbreaks of HIV, is that the virus primarily spread in these communities via sex and drugs.
Next: A few remarks on the contemporary landscape of sexual, injection, and transfusion-based risk.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Suitably summarized on wikipedia: https://en.wikipedia.org/wiki/Vaccine_contamination_with_SV40
Shah, K. Nathanson, N. (1976.) “Human exposure to SV40: review and comment.” Am J Epidemiol. 1976 Jan;103(1):1-12.
Or later — to recycle a previous footnote, I haven’t verified the timespan during which the FHHBV was prepared by targeting gay men as donors. In
Pépin, Jacques. The Origins of AIDS Cambridge University Press. Kindle Edition.
Pépin references print and TV ads for plasma donors for the FHHBV targeting gay men in July, 1981, just after the vaccine was licensed.
Sweet, BH. Hilleman, MR. (1960.) “The vacuolating virus, S.V. 40” Proc Soc Exp Biol Med. 1960 Nov:105:420-7.
It is never a bad time to point out that a successful anti-“pandemic” vaccine was made in under a year half a century before the mRNA vaccines. The relevance of this vaccine was a bit diminished by the finding that most older people had pre-existing natural immune recognition of H2N2.
e.g. Hilleman, MR. (1995.) “Overview: practical insights from comparative immunology and pathogenesis of AIDS, hepatitis B, and measles for developing an HIV vaccine.” Vaccine. 1995 Dec;13(18):1733-40.
An entire textbook could be assembled out of his late papers — each one contains multiple references to his own solo work on related topics.
Unlike many vaccinologists, his legend does not begin with an early trauma that informs his lifelong pursuit of revenge against viruses. Still I doubt it ever occurred to him that viruses have any sort of on-balance positive effects for humans; that sparing a trainable pattern-recognition system from training in its early years seems unwise from an evolutionary perspective, or at least invites a prediction of futility in terms of individual human betterment. In his last years he was imperially dismissive of the same nascent anti-vaccine movement spurred on by the rampant expansion of the vaccine schedule which society at large had not actually asked for.
Buynak, EB. Roehm, RR. Tytell, AA. Bertland 2nd, AU. Lampson, GP. Hilleman, MR. (1976.) “Vaccine against human hepatitis B.” JAMA. 1976 Jun 28;235(26):2832-4.
Via inoculation of marmosets.
Via inoculation of chimpanzees during testing.
This may be because principle investigator Wolf Szmuness, whose interest in Hepatitis B followed a blood-transfusion-infection of his wife, and who was earliest to organize surveying and screening efforts in preparation for vaccine trials, was to die of lung cancer in 1982. It is notable that Szmuness’s wikipedia page (accessed October 9, 2023) includes mention of the Hepatitis B vaccine HIV theory. At all events the New York city trial in gay men was published and included in Hilleman, et al.’s 1983 summary of clinical use of the vaccine, but nothing is cited in the same regarding the trials in dialysis centers. All I have located is a limited report on one center (with preceding low case rates), involving 79 patients (this paper however numbers overall trial patients as “nearly 900”):
Stevens, CE. Goodman, AI. Szmuness, W. Weseley, SA. Fotino, M. (1980.) “Hepatitis B vaccine: immune responses in haemodialysis patients.” Lancet. 1980 Dec 6;2(8206):1211-3.
Bergamini, F. et al. “Immune response to hepatitis B vaccine in staff and patients in renal dialysis units.” J Infect. 1983 Jul:7 Suppl 1:35-40.
Milne, A. Dimitrakakis, M. Allwood, G. Lucas, R. Moyes, C. Pearce, N. “Immunogenicity of low doses of hepatitis B vaccine in children: a study in 650 New Zealand children.” J Med Virol. 1987 Dec;23(4):401-5.
SUBJECTS AND METHODS
Six thousand children had been vaccinated by nonmedical staff in a community funded programme. Children aged less than five years were not tested for HBV markers before vaccination; older children had been tested for anti-HBs and HBsAg. In the Borough of Whakatane, 1,510 children had been vaccinated as part of the overall programme. It was decided to carry out postvaccination testing in preschool (<5 years) and schoolchildren in Whakatane Borough, since this community (population 13,000) was comparable in key respects to that of the town of Kawerau where children had been given low doses of H-B-Vax at 0, 1, and 2 months. Parents were contacted, seeking informed consent to testing of their children, and 670 agreed. Blood samples were taken from children at schools and special clinics 2-3 months after the last dose of vaccine. Serum was separated within 24 hours of collection and stored in polypropylene tubes at minus 35OC until tested.
Plasma-derived vaccine (MSD H-B-Vax [— the FHHBV]) was used. Each 1.O ml vial contained 20 mcg. A 0.1 ml (2 mcg) dose of vaccine was given in the upper deltoid muscle using insulin syringes, BD type U100.






https://youtu.be/PnJ5T1Enwq4?si=GwfcOK7qLpWMLw8s
This is interesting in a sideways way. Dr John Campbell ( PhD Nurse Educator) interviewed Professor Dalgliesh about an all round vaccine - injecting mycobacterium to stimulate the immune system to up the T cell activity which then kills off other infections and cancers. In passing he mentions his previous work with HIV and I think he said that the human HLA status determines how susceptible people are to progression on to AIDS. I think he said that people who were HLA-B27 could have a huge amount of HIV in their blood and still not go on to develop AIDS. He said the monkeys were all HLA-B57 ( I think - I was gardening at the time of listening to this at double speed!) and said that all the other types presumably died out due to susceptibility to the SIV virus.
You might be interested in why are they really pushing Hep B:
https://scientificprogress.substack.com/p/vaxxed-v-unvaxxed/