Post-mRNA IgG4 does not raise overall IgG4
Also, 26% of a small sample of recipients have ~10-fold higher (!) proportion of anti-spike IgG4 B Cells by the third dose
Summary:
A new preprint replicates previous findings of post-mRNA Covid vaccine IgG4, without expanding the window of observation (mid-2022)
A previously unanswered question:* Does abnormally high anti-spike IgG4 resulting from the Covid vaccines increase overall IgG4 (probably not, but would someone measure this)?
The new preprint measures this. Overall IgG4 is not increased very much. (Therefore concerns generically related to diseases involving high overall IgG4 are probably not warranted, pending future changes.)
*I belatedly have realized that Irrgang, et al. already showed overall B Cell IgG4 proportions to be low in vaccinees, as I reported at the time. Oops. However, this new study gives us a more satisfying before-and-after-comparison.
More alarmingly, 5 of 19 donors in the Pfizer/mRNA-only group experience nearly 10-fold IgG4 conversion as their peers by the 3rd dose. Age and breakthrough infection do not seem relevant, suggesting a random process (i.e. vaccine variability).
Finally, the authors propose that excessively rapid second-dose timing may contribute to IgG4 (the mRNA schedules were shorter than the AstraZeneca schedule was).
More of the same: IgG4 after 2nd and 3rd mRNA dose
The glacial progress of updates regarding post-mRNA conversion of anti-spike B Cells to producing IgG4, which neutralizes the virus but potentially blunts cellular immune response to infected cells (unless antibodies to other viral proteins get the job done in their place), continues. This time with a preprint from Tuesday belatedly describing results from blood draws taken in July, 2022.1

Background
This adds to and reinforces results from three prior studies which have interrogated IgG4 directly2: mRNA vaccinees begin to convert to IgG4 after the second dose; there is something of an advancement after the third dose; this doesn’t take place in individuals with mRNA boosters following an adenovirus (AstraZeneca) primary series. Since these results are not novel, the reader may wish to simply visit the review offered here of Buhre, et al., the “second” study.
Or, for an introduction into the problem, see as always my original report on Irrgang, et al., the “first” study.
Finally, the use of samples taken in July, 2022, makes this new preprint not much more up-to-date than Adhikari, et al. (reviewed here), the “third” study, which confirmed that IgG4 is included in maternal antibodies passed on to newborns (which will fade after a few months).
And in synthesis, my series on tolerance and severe disease discusses possible implications for future infections:
Results (that are the same as already known)
With those guides offered, we may observe the results in this new preprint and find that there is nothing surprising: Within the pool of memory B Cells and antibodies directed toward the spike protein, IgG4 appears after mRNA primary series (and booster) (“homologous” donors), but not after adenovirus series and booster (heterologous” donors). The following pools all donors from each group, and shows the emergence of IgG4 6 months after dose 2 (exactly as originally evident in the “first” IgG4 paper). We are looking at spike-positive memory B Cells, and considering what percentage of them are for what class of antibody (wizard, berserker, assassin, healer?, essentially).

Results for antibodies are similar, because these B Cells make antibodies (alongside with some other cells that won’t be found in the blood). Beyond the group results there is considerable individual variation in the individual IgG4 dynamics for mRNA-only donors, as shown below.
A bit of new: Overall IgG4 not obviously altered; individual super-converters; vaccine timing involved?
i. Overall IgG4
A pending question3 has been whether the abnormal conversion to IgG4 for anti-SARS-CoV-2-spike B Cells and antibodies leads to an altered composition for all antibodies (or memory B Cells). In other words, does it change the big picture of what kind of antibodies are found in the blood, or is it just a drop (called “anti-spike antibodies”) in the immunity ocean. The latter seemed more likely, but Hartley, et al. offer more direct confirmation. From the supplemental materials — again, this looks at Memory B Cells, which produce some of the antibodies that circulate in the blood all day long4:
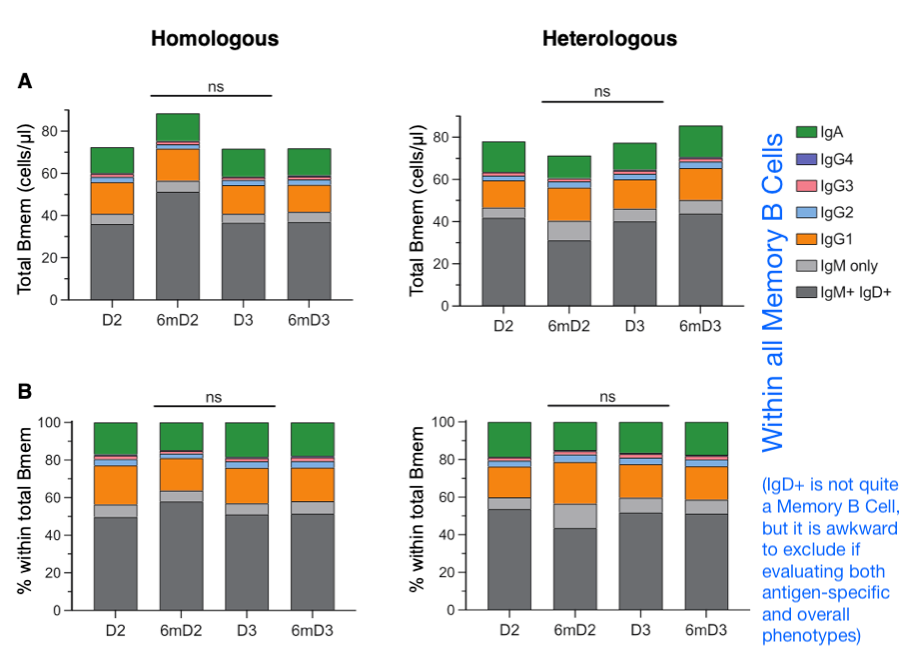
Thus, we have an answer: No big change in overall IgG4 production.
ii. 5 of 19 mRNA-vaccinated are super-converters
Finally, and keeping the focus on donor circulating Memory B Cell classification, here are individual results. Previous results (including the “first” IgG4 paper) have already hinted at substantial variation in IgG4 conversion after mRNA vaccination; but focusing on B Cells offers a less noisy way to measure IgG4 proportion.

The takeaway here is that 5 of our 19 mRNA-only donors are substantially more IgG4-converting by Dose 3, seemingly 10-fold of the average in proportion, and for all but 1 this reflects a “head start” after the second dose (with only one head-starter failing to progress further). By 6 months after the 3rd dose, these individuals seem to be nearing IgG4/IgG1 parity.
Age and breakthrough infection do not seem relevant for this trend — if this finding extends to all recipients (perhaps due to some individuals receiving far more in-tact mRNA in their vaccine than the norm, as previously proposed here), then for 25% of mRNA recipients, IgG4 conversion is extreme.
As discussed before, these results are a glimpse of a trend measured well in the past — it can only continue to ratchet toward the same direction. IgG4 is the “last stop” in Memory B Cell class switching, and cannot be reversed (the failure of new anti-spike B Cells to swoop in and rejuvenate the pool has now been shown in multiple studies, discussed here). From my review of Adhikari, et al., which sampled mRNA recipients up to May of 2022:
iii. Finally, RE vaccine timing
As a last measure of novelty, Hartley, et al. propose that the distinction between mRNA and adenovirus vaccines — why only the former induces IgG4 — could reflect differences in injection timing in the primary series (which of course was chosen completely arbitrarily, with no human studies completed to compare different injection regimens during Warp Speed). It is a notable suggestion — captured in their figure 1:

In their own words, “IgG4 expansion in the homologous group is either due to the timing between dose 1 and 2 or the primary vaccination formulation” — a simple statement of what is potentially different about the two platforms. Further, “Examining a cohort of individuals who received a primary mRNA vaccination with a longer duration between doses 1 and 2 would confirm whether this effect is due to dosing or vaccination formulation” — presumably satire, as everyone knows you are not supposed to research how vaccines actually work in humans.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Irrgang, P. et al. “Class switch towards non-inflammatory IgG isotypes after repeated SARS-CoV-2 mRNA vaccination.” Sci Immunol. 2023 Jan 27;8(79):eade2798.
Buhre, JS. et al. “mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine.” Front Immunol. 2023 Jan 12;13:1020844.
Adhikari, EH. et al. “Diverging maternal and infant cord antibody functions from SARS-CoV-2 infection and vaccination in pregnancy.” biorxiv.org
Already partially answered in Irrgang, et al. and reported by me last year, I belatedly remembered after posting. Again, regarding overall B Cells, this new paper just offers a more thorough sampling of before and after.
In fact, arguably a problem would show up in circulating Memory B Cells first, since long-lived plasma cells in bone marrow might (might) skew overall antibodies toward earlier immune exposures, as far as I know. Overall it is probably a cleaner way to measure what is going on.








This is fascinating that they think the length of days in between is the most likely reason instead of the mRNA brand new technology... cant taint that mRNA technology when there are so many possible lucrative products they can still shove it into and plow into the masses..
"The new preprint measures this. Overall IgG4 is not increased very much. (Therefore concerns generically related to diseases involving high overall IgG4 are probably not warranted, pending future changes.)"
But what about Fc-Fc binding, and the associated ability of these IgG4 antibodies to neuter the ability of good antibodies to take the fight to...let's say... a small incipient cancer? My contention is that a little IgG4 can go a long way. Why? Because IgG4 doesn't do it's Fc-Fc damage to the sea of antibodies floating around in the general population....no, it does this non-specifically to those good IgG antibodies that have found, and bound to, their target.
https://journals.aai.org/jimmunol/article/182/7/4275/81101/Human-IgG4-Binds-to-IgG4-and-Conformationally
"IgG4 binds to solid-phase bound IgG1, but solid-phase bound IgG4 does not bind IgG1
Previously, IgG4 Fc binding was observed in assays with Fc or intact IgG coupled to a solid support (6) or to IgG subclasses in an immunoblot (9). In Fig. 2,A, we compared binding of 125I-labeled IgG1 or IgG4 to Sepharose-coupled IgG1 or IgG4. IgG4 binds both to coupled IgG1 and IgG4. Binding to IgG1 appears to be slightly more efficient, but IgG4 Fc binding during the coupling of IgG4 could reduce the capacity of the IgG4-Sepharose. Surprisingly, IgG1 does not bind to Sepharose-coupled IgG4, which implies the binding activity of IgG4 to be restricted to IgG coupled to a solid support. This suggests that IgG4 Fc-binding activity is directed either against epitopes that become available only after coupling...."