Post summary (click to expand):

*Update, February 2, 2023: A third paper has now been uploaded to preprint affirming the same result:
Despite the extreme redesign of the spike protein in BA.1 (and other Omicrons), infection does not prompt the generation of new (naive-derived) B Cell clones. Instead, vaccine-derived (and likely IgG4-converting) B Cells merely reshuffle and remodel to generate cross-reactive (but likely still IgG4-biased) antibody recognition.
Boosting Mice to (Tolerance-Mediated) Oblivion
A study reported by Alex Berenson1 replicates tolerance in mice with repeat-injection of recombinant spike protein Receptor Binding Domain.2
This didn’t strike me as too informative, at first; it wouldn’t be surprising if tolerance could be induced with anything injected into mice six (not four) times. Stop injecting whatever same thing into those mice over and over! And, we already know the craze of topping up antibodies with “boosters” was reckless and pointless from the start. Antibodies are supposed to wane; if they weren’t, they wouldn’t.
Potentially revealing, is that Gao, et al. verify makers of tolerance and exhaustion in T Cells in the six-dosed mice. These results may or may not apply to spiked-overdosed mRNA Covid vaccine recipients (of two or more doses); and if applying, may be only temporary. With that said, they are consistent with immune dysregulation in chronic disease:3
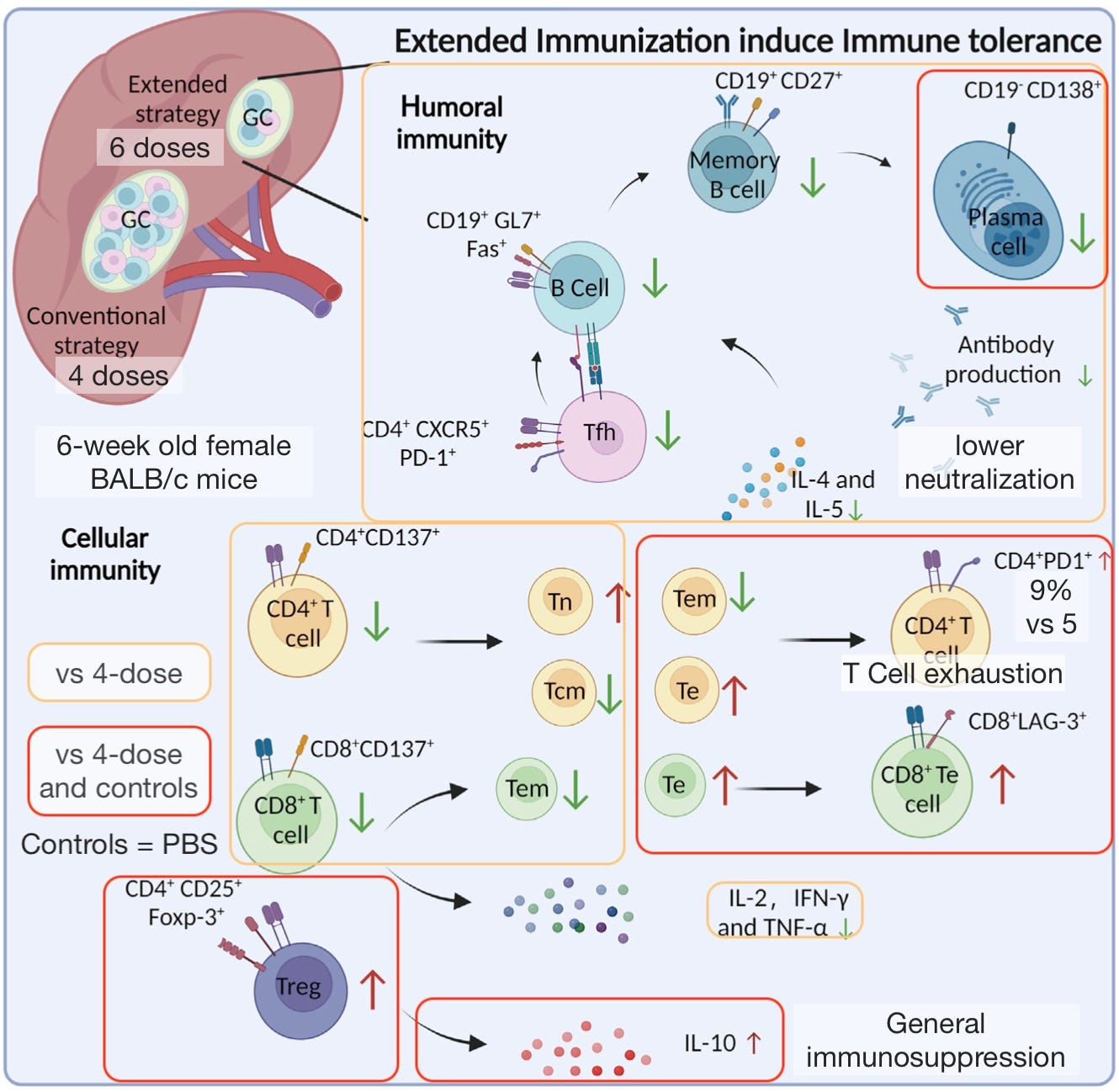
The distinction between tolerance and exhaustion may be more about the “mood” that we imagine T Cells to be invoking, but generally the latter suggests itself as more a marker of chronic infection and inflammation and generic immunosuppression.
In other words, excessive antigen exposure alone appears to lead to (short term? long term?) generic immunosuppression. In a module not represented in the image above, the 6-dosed mice T Cells were less responsive to stimulation with HPV virus peptides vs. controls; unsurprising given that more cells would have been “exhausted.”

As before,4 I would suggest diabetes as a conservative touchstone for the likely degree of immune suppression that such chronic-inflammatory-associated responses would represent, should the findings in Gao, et al. apply to Spike Overdosed Humans (aka mRNA transfection recipients).
No B Cell Calvary for IgG4 Converters after Omicron infection?
In a previous look at B Cell responses after Omicron “breakthrough” infection, it was found that:5
Post-infection antibodies were able to bind against Omicron just fine.
The Memory B-Cells making these antibodies were all cross-reactive to the original (vaccine variety) Wuhan spike.
The lingering question, in light of the discovery of IgG4 tolerance, was whether some new Memory B Cells would arrive that were “specific” to Omicron later on. This does not appear to be the case.
Context: My Old School
(For prior discussion of IgG4 after mRNA Covid vaccination, see “Tolerance Cometh.”)
Even before the discovery of what is now called cellular immunity, it was observed that antibodies to new strains of viruses tend to cross-react with previous strains, but not vice versa. With modern tools, we can replicate this observation by shooting B Cells through tubes and seeing whether their B Cell Receptors (which represent the antibodies they can produce) are sticky for just one or the other, or both of two different variants of a viral antigen.
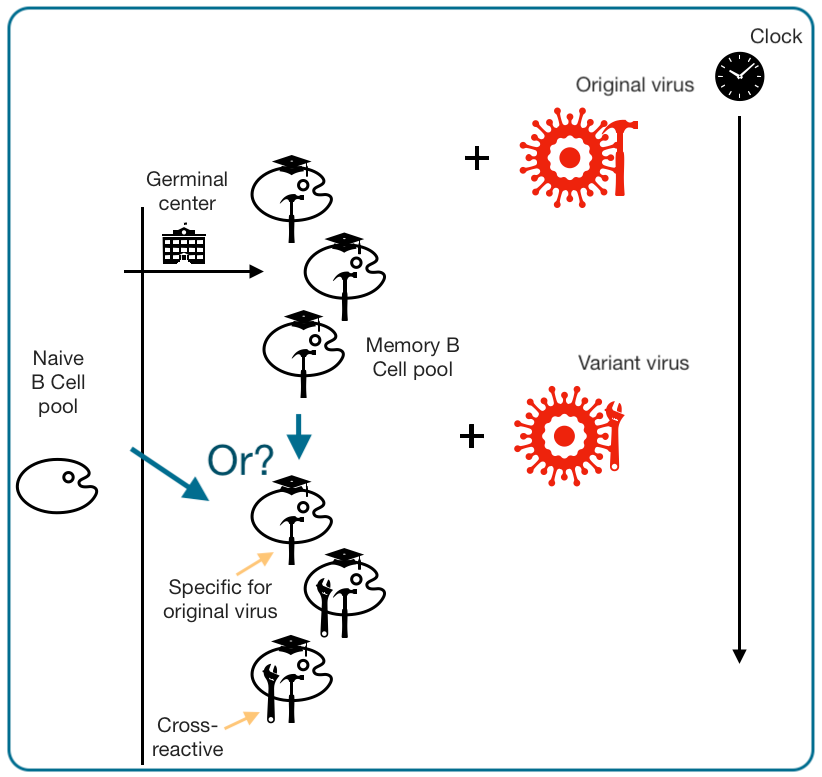
What often tends to be found is that after exposure to a variant antigen, the memory B Cell pool goes from having cells that are mostly good at binding the original version, to having cells that are good at binding both versions (and a few leftover that are just good at the original version).

The question becomes whether that second group of B Cells came from new, naive B Cells going off to Germinal Center School, or if it is the case that the prior pool of graduates simply became “re-skilled.”
The answer typically turns out to be the second option: Existing B Cells re-skill themselves, either by immunodominantly re-entering germinal centers to outcompete naive cells in affinity maturation, or re-shuffling clonal distribution to bring B Cells “from the back bench” onto the court, or both, etc.
It’s a mundane answer, typically, though it has been broadly catastrophized under the brand-name “imprinting” (it might better be referred to as “immune plasticity;” you can form a new response from your old response).
With IgG4 conversion, this mundane feature of the immune system potentially becomes a problem.
With the mRNA vaccines, the original Memory B Cell pool, presumably due to excessive encounter with the spike protein, goes from a chipper gang of fresh graduates to jaded teens who want to “tune out” complement-mediated and cellular immune responses to spike.
After infection with one of the Omicrons, this pool of B Cells will re-shuffle and remodel to be cross-reactive to both Omicron and the original vaccine spike; but they won’t stop being programmed for IgG4. IgG4 B Cells cannot “revert back” to a different model of IgG as they have literally thrown away the required genes.
The only way to tamp down on tolerance thus may be for new, naive B Cells to enter germinal centers and join the pool, contributing (literal) “fresh blood.” Otherwise, even though the transfection-trained B Cell pool can “learn new spike tricks,” it will still prefer to tell the immune system to ignore spike.
Quandt, et al. found that Omicron-binding B Cells sampled 44 days after Omicron infection were almost universally also Wuhan-binding — in other words, they were just members of the “re-skilled” post-vaccine B Cell pool (IgG4 was not examined).6
This left the question of whether, maybe, new B Cells would nonetheless show up later, as is frequently found in sequential flu exposure studies that bother to wait more than 11 days before sampling.7
Two new studies (ok one is from September and I just found it) affirm this finding. The mRNA-vaccinated are (mostly) stuck with the original B Cell class.
Study 1: No luck at 6 months
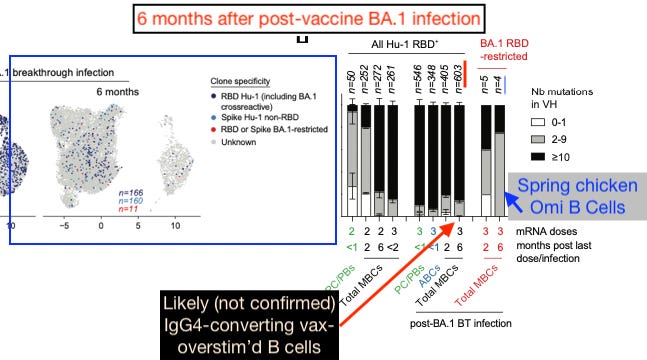
Kaku, et al. sampled seven Moderna-vaccinated donors after their BA.1 infections, first at 14 to 27 days and then at four to six months.8
For the four donors whose samples were evaluated in flow cytometry (swimming through a tube while sticking to spikes) did a huge new rush of Omicron-specific Memory B Cells appear, to rescue the participants from their (we will presume) IgG4 conversion treadmill (IgG4 was not measured)?
No. B Cells were primarily cross-reactive, suggesting that the original pool was still dominant.
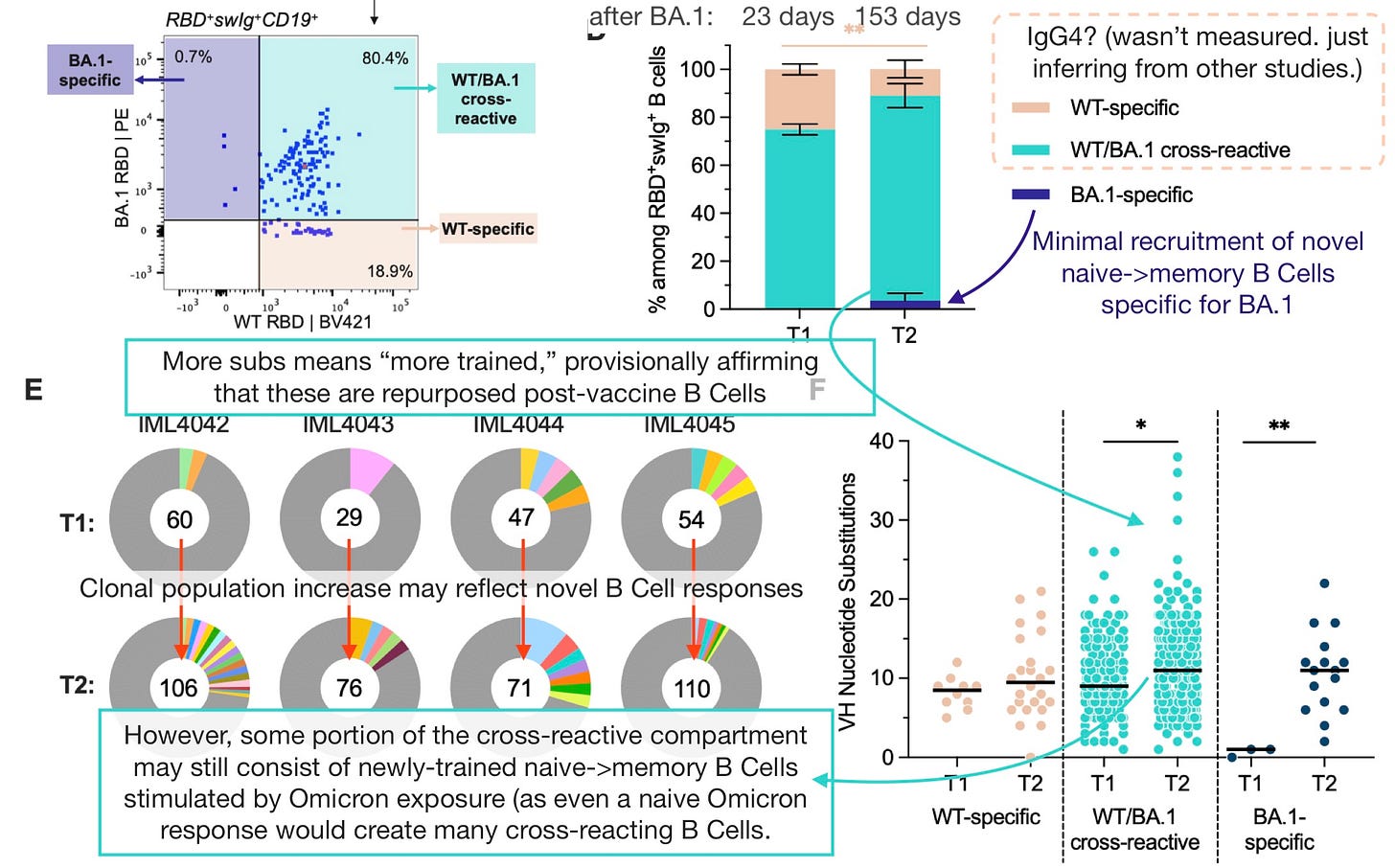
The picture on an individual basis revealed that two donors were well below the average:

Alternate interpretation: Balanced response?
However, this all falls short of validating that the cross-reactive (turquoise) B Cells are, in fact, from the original (presumably IgG4-converting) Covid-vaccine pool. Notably, at 6 months (T2), all donors are close to parity in Wuhan and BA.1-specific B Cells, suggesting the immune response is balanced.
Thus, despite no clear signal in the form of a really “young” (low-VH-mutation) B Cell distribution, it could be that something like half of these are new, BA.1-stimulated B Cells from the naive compartment that just happen to cross-react. In which case these donors would be halfway free of the IgG4 B Cells (proportionate to all B Cells that will make antibodies that bind to future encountered spike protein).
It would be more useful, obviously, to have a study measuring long term post-infection IgG4 directly.
Study 2: No luck after two Omi exposures (infection + bivalent), or bivalent alone.
Addetia, et al. looked for Omicron-specific B Cells following combinations of either (post-vaccine) Omicron infection or BA.1 or BA.5 “bivalent” mRNA injection.9 Few were found (red is either "Omicron-pool" or BA.1-specific; there are more hits for the cohorts tested with the "pool").
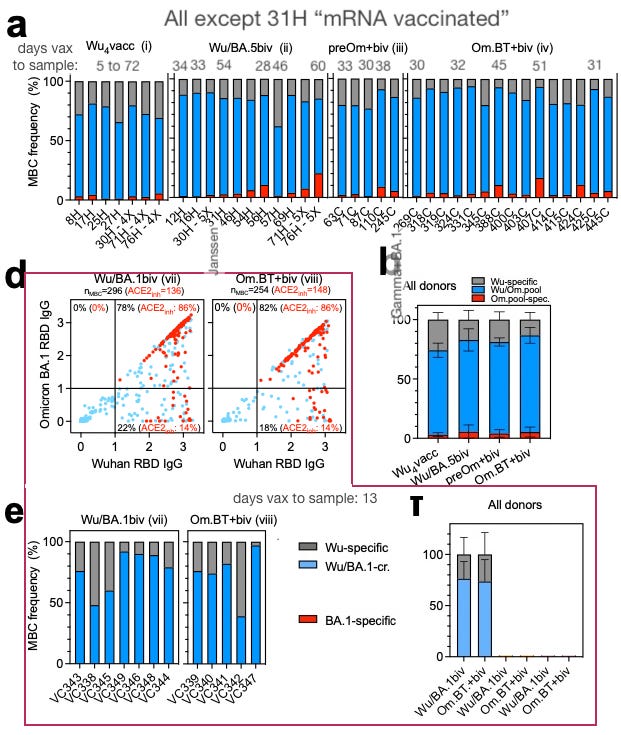
Takeaways:
It’s not clear if more time increases the Omicron-specific response post-bivalent injection. Generally, draws reflect that after the medium term, there’s very little difference from the Wuhan-boosted (“Wu4”).
Generally, “double-exposure” doesn’t seem to move the needle from cross-reactive to new, specific B Cells in either cohorts iv or viii.
Meanwhile, the “Wu4” group, which represents four injections of the original recipe, may be more evidence that the original vaccine front-loads “mutant” spike B Cell responses. This front-loading could explain why “imprinting” seems to be the rule for the Covid-vaccinated, as I speculated in my review of Quandt, et al.10
More suppressed cellular immunity?
Addetia, et al. did not look at IgG4; but they did poke around to see how well donor plasma bound to “FcγRIIIa.” This is essentially asking plasma how well it will theoretically tell Natural Killer and T Cells to destroy cells that have spike protein. Omicron infections (cohort viii) don’t seem to improve this stat one bit.

The best-off are those who had a pre-Omicron infection (it is not clear whether this was before or after the original course). It is an interesting finding. These individuals are sampled shorty after the third dose; but so were those in the “Wu3” group. It could be that the “preOm” group were all infected before their original mRNA vaccination, and are being protected from tolerance by this original response — which would match the results found in Buhre, et al.:
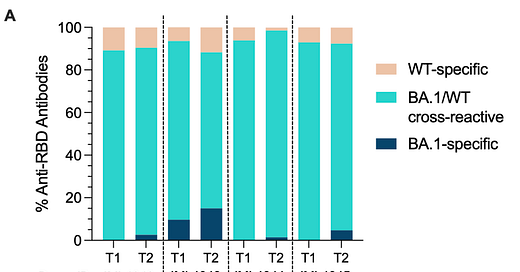
Study 3: 6 month failure replicated
Not much needs to be said about Sokal, A. et al.11 As with the other two papers, it did not look at IgG4 directly. It does go a bit further in characterizing how the remodeling of the previous B Cell repertoire reflects a fluid, competent response, not simply "fixation" on the originally encountered spike as in Thomas Francis's peevish characterization.
And so “imprinting” would be simply one more marvel of our naturally-evolved immune system, but for the problem of IgG4 induced by mRNA spike overload.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Berenson, Alex. “VERY URGENT: After four shots, Covid jabs sharply REDUCED immune function in mice.” (2023, January 18.) Unreported Truths.
[It’s six injections, not four.]
Gao, FX. et al. “Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice.” iScience. 2022 Dec 22;25(12):105479.
Dong, Y. et al. “CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B.” BMC Immunology volume 20, Article number: 27 (2019)
See footnote 5.
Kaku, CI. et al. “Evolution of antibody immunity following Omicron BA.1 breakthrough infection.” biorxiv.org.
Addetia, A. et al. “Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants.” biorxiv.org
See footnote 5.
Sokal, A. et al. “Omicron BA.1 breakthrough infection drives long-term remodeling of the memory B cell repertoire in vaccinated individuals.” biorxiv.org









So basically the equivalent of allergy shots....they've desensitized the population to covid. Wonderful.
All these people can still catch covid,
They can still spread covid,
And it continues to damage their organs (unlike allergy shots which only desensitize you from something harmless like pollen).
People have been turned into ticking tome bombs of death.
Walking weapons of mass destruction.
Well, put a psychopath in charge and that's what you get.
Why be concerned about so called variants when the message is loud and clear since more than a year if it was not so before then, that the mRNA shots will not prevent expression of the Covid-19 symptoms nor possible transmission to others. In addition since the earliest days of the rollout, worldwide evidence was gathering quickly and giving a strong signal that multiple shots may lead to severe depression of the body's ability to deal with ANY invasive pathogen, even to near zero capability in an AIDS like condition.