It's Probably Not an Alzheimer's Virus
A calming talk sheds light on the links between infections, innate immunity, and Alzheimer's.
A fresh interview by Amy Proal stands as excellent viewing on its own and, perhaps more importantly at the moment, as a calming antidote to the currently-raging Amyloidic Panic.
If the reader is not aware of the Amyloidic Panic, suffice it to say that magical powers are being ascribed to the spike protein in certain corners, without much in the way of coherence or context. The latter - context - is especially important. It grounds every observation about a given element in biology by asking the question, compared to what? It may turn out that for any observation about SARS-CoV-2 or the spike glycoprotein, everything else in the category “virus” or “viral glycoprotein” also would prompt the same observation; we humans just haven’t gotten around to looking yet.1
The interview below should offer the viewer a better sense for how likely this is to be the case when it comes to the question of a link between SARS-CoV-2 and amyloid formation.
By the brilliant Amy Proal, with the charismatic and absurdly still-youthful Alzheimer’s research legend Rudolph Tanzi, on his “antimicrobial protection hypothesis”:
Highlights 1: The Ubiquity of Amyloid
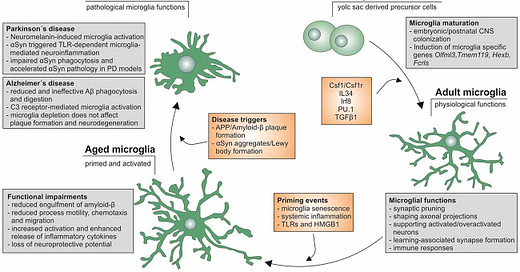
4:00 The amyloid plaques that characterize Alzheimer’s disease are a feature of the brian’s innate immune system. Amyloid-beta/β peptides are the brain’s version of evolutionarily ancient antimicrobial innate immune peptides, bearing a resemblance to other known endogenous antimicrobial peptides including human LL-37.2 Amyloid-β sticks to microbes, quickly (12:20) forming plaques that trap them and prevent their proliferation in the brain. Which is, you know, good.
39:00 Every microbe investigated so far seeds the formation of amyloid-β plaques.
8:20 Plaques promote Tau protein neurofibrillary tangles; tangles disseminate amyloid plaque and prompt inflammatory response from microglia (the innate immune cells that act as housekeepers and sentinels in the central nervous system). (13:30) Amyloid-β (again) induces tangles; and (14:20) tangles also disseminate to nearby neurons to promote more tangles. (14:45) These redundancies are essential since the brain relies exclusively on the innate immune system to keep microbes in check.3
12:55 Tangles are also spontaneously promoted in neurons by viral encounter, as demonstrated with Herpes (not yet published4). Tao protein tangles prevent egress of viruses along the axon.
19:55 Plaques and tangles precede onset of Alzheimer’s symptoms by 30 years. Think of plaques as the match; tangles as brush fires - it takes 30 years of these brush fires before the forest fires of neural inflammation cause enough cell death to prompt symptoms.
38:25 Is SARS-CoV-2 more likely to promote plaque-formation than other infections?
It probably promotes some plaques and tangles, since every microbe we have looked at does.
A study of post-mortem brains of younger diagnosed Covid-19 patients showed high levels of amyloid and tangles (perhaps not, actually5).
And the Spanish Flu may offer a precedent of a pandemic followed by late-onset neurological disease for those young at the time.
40:50 However, SARS-CoV-2 does not proliferate in the brain; it is not neurotropic, thanks to the scarcity of ACE2 in nerve cells. Compared to herpes viruses, it probably features less of a presence and thus less innate immune (Amyloid-β) response. It probably promotes some plaques and tangles on its way to being stopped.
41:45 In at least some post-mortem brains, there were high levels of plaques. But we have to ask whether this was a pre-existing condition or a reflection of a pre-existing condition, that led to worse outcomes from infection.6
42:05 As for Long Covid, nicotinamide riboside (NAD+) is showing promise in an ongoing trial for clearing brain fog. NAD+ increases the availability of ATP. Microglia (and other macrophages) have both a housecleaning role and a sentinel/destructive role; the former requires more energy. We are finding with Long Covid that the inflammation comes from the blood vessels of the meninges around the brian. Whether they are being stimulated by inflammation, a pathogen (PAMP), or cellular debris (DAMP), providing the microglia (and other macrophages throughout the body) with more ATP helps coax them back into housecleaning mode, including increased clearance of amyloid-β during sleep.7
Note that Tanzi’s point about SARS-CoV-2 not being neurotropic doesn’t address the more specific question of “spike as a toxin.” Tanzi makes no mention of free-floating S1 (the “bulb” of the spike protein, which often breaks off and enters circulation during infection or after Covid vaccination8) or other viral proteins which may circulate freely during infection, and whether they may promote a amyloid-β response despite lack of replication in the brain. However, I think the (likely) references for his comments on post-mortem brains offer evidence that there is no widespread penetration of the brain by viral proteins or virions during infection.9
Further perspective on the “ubiquity of amyloid” is provided by Tanzi and co.’s 2018 overview:
Moir, R. Lathe, R. Tanzi, R. (2018.) “The antimicrobial protection hypothesis of Alzheimer's disease.” Alzheimer's & Dementia. Volume 14, Issue 12, December 2018, Pages 1602-1614.
Features of amyloid-β assumed to be intrinsically pathogenic - such as oligomerization and fibril formation - are in fact an intrinsic feature of antimicrobial peptides in general. They are how the innate immune system “does the work” of limiting the movement of microbes:
The broad role for oligomerization [bundling of peptides or proteins] in normal AMP [anti-microbial peptides] function suggests that the propensity of Aβ to form oligomers may not be the intrinsically abnormal behavior portrayed in prevailing AD [Alzheimer’s Disease] models. Instead, Aβ oligomerization may normally mediate antimicrobial actions, as is the case with established AMPs. Consistent with a key role for oligomerization, the specific in vitro antimicrobial activity of cell-derived and synthetic Aβ oligomers [so, Amyloid-β peptides that were bundled together] is at least two orders of magnitude (log10) [i.e. 100X] higher than monomeric [non-bundled] in vitro prepared synthetic material [5], [6]. Furthermore, we have recently shown that Aβ oligomerization mediates binding, anti-adhesion, agglutination, and entrapment activities against microbial pathogens.
Highlights 2: The Developing Picture for Alzheimer’s
9:00 Does that mean “infections cause Alzheimer’s”? The answer is complicated from a clinical perspective. (17:50) Our comparison of Alzheimer’s patient post-mortem brains and healthy controls (age-matched or younger) found no smoking gun.10 We did observe that younger brains had bacteria associated with healthy gut microbiomes, but not older brains.11 (23:20) Another method is to use lasers to remove plaques and examine genetic material within, or trawl for patterns in Memory B Cell repertoires.
9:00, 32:15 Genetic propensities for higher production or lowered clearance of amyloid plaques may confer a fitness benefit against infections that normally lead to lethal encephalitis (better innate suppression). Caleb Finch’s research suggests that within tribal populations, individuals with the Alzheimer’s-associated ApoE 4 allele may survive to old age more frequently.12
21:00 Tangles are also directly caused by head trauma, as from repetitive impact sports, and still lead to neurological disorders on about the same timeline as we suggest for infection-prompted “brush fires.”
25:35 Could air pollution, which also prompts the formation of amyloid-β plaques, be driving later development of Alzheimer’s? Like concussions, it may be one of the seeds leading to eventual microglia-mediated neuron destruction. (29:20) Progress in understanding chronic disease requires understanding that each individual has their own unique pathway to disorder, as opposed to a binary (Koch’s Postulates) exposure/disease model.
30:15 Likewise, having the ApoE 4 is the biggest genetic risk factor for Alzheimer’s, but doesn’t guarantee the disease or determine the age of onset; so other lifestyle factors and environmental or infection factors still drive the story.
31:10 For lifestyle, Tanzi emphasizes “SHEILDS”
Sleep: Daily clearance of amyloid.
Handling stress: Reducing daily production of amyloid.
Interacting with others
Exercise: Clears amyloid and reduces inflammation.
Learn new things: Learning generates new synapses, adding “money to the bank” of how much you can afford to destroy.
Diet: Healthy gut microbiome keeps amyloid down and reduces inflammation.
For further reading on the question of whether infections “lead” to Alzheimer’s, or if the question remains more one of how aging drives higher inflammation to normal stimuli (including amyloid plaques), see:
Abbott, Alison. “Are infections seeding some cases of Alzheimer’s disease?” (2020, November 4) Nature (news feature).
For a further meditation on the likelihood of confusing previously unknown normal viral properties for unique super-virus mechanisms, see “The Biobank Brain Change Study.”
LL-37 is described at https://en.wikipedia.org/wiki/Cathelicidin.
Note the intrinsic resemblance to clotting pathways, which have built in positive feedback cycles involving inflammation.
For Amyloid-β generation by Herpes virus, see:
Eimer, W. et al. “Alzheimer’s Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection.” Neuron. Volume 99, Issue 1, P56-63.E3, July 11, 2018
Tanzi is seemingly referring to:
Rhodes, C. et al. “Β-Amyloid Deposits in Young COVID Patients.” The Lancet. (preprint).
However, note that deposits did not stain as truly amyloid (hence the avoidance of the term “plaques”), and no Tau tangles were found. The authors suggest that these deposits are likely indirectly mediated by hypoxia rather than directly by the infection itself.
Here perhaps referring to:
Poloni, T. et al. “COVID‐19‐related neuropathology and microglial activation in elderly with and without dementia.” Brain Pathol. 2021 Sep; 31(5): e12997.
Note that plaques were found mainly in the brainstem, as opposed to prefrontal cortex (the normal site for Alzheimer’s-associated plaques).
So, an indirect answer overall. Tanzi doesn’t appear fully confident that SARS-CoV-2 is not plaque-forming; but if it is, then he believes the microglial response can be targeted to convert from a destructive to ameliorative state.
Ogata, A. et al. “Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients.” Clinical Infectious Diseases, Volume 74, Issue 4, 15 February 2022, Pages 715–718.
Since Rhodes, C. et al. found that Amyloid-β did not form true plaques, it does not support dissemination of the virus or viral proteins in the brain. Since Poloni, T. et al. found that microglial activation was limited to the brainstem, it suggests that exposure is limited and does not derive from widespread crossing of the blood-brain barrier by S1.
I was not able to find any publication corresponding to this work.
Proal did not ask the obvious question of whether gut microbiome disruption (including via over-prescription of antibiotics among the elderly) could be driving poor brain health or more rapid brain aging. For more, see “The War on Pee.”
Vasunilashorn, S. et al. (2011.) “Inflammatory Gene Variants in the Tsimane, an Indigenous Bolivian Population with a High Infectious Load.” Biodemography Soc Biol. 2011; 57(1): 33–52.
Van Exel, E. et al. (2017.) “Effect of APOE ε4 allele on survival and fertility in an adverse environment.” PLoS One. 2017; 12(7): e0179497.



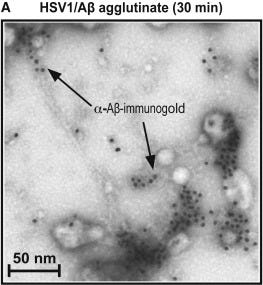



So, then, the answer is "this is complicated", right? I know a large number of people who had Covid and have not changed cognitively.
That said, another side of this is formation of fibrous clots in blood, which does seem to have plenty of confirmation in literature about Covid and embalmer reports and videos.
I consider the amyloid theory as something interesting that I need to follow with open mind.
"I think current investigations into the virus and the Covid vaccines don’t really offer either the writer or the reader a high 'return on time-invested - compared to, you know, just waiting and seeing what happens in the coming months."
Spot on.
My father had a saying: "Time heals and time reveals."
He stated it in the context of relationships. But, its probably apropos to Covid and the vaccines. Time may reveal that these vaccines are the biggest medical blunder/disaster in history. Or, time may heal the injuries and compromised immune systems of the vaccinated. Regardless of what it will reveal and whether it will heal, continued analysis and speculation at this junction in time would not be very productive. We will have more evidence and data soon enough. In the meantime, as you expressed, we need to live our lives.
Looking forward to your next post, whenever there is something pertinent to write about.