Tolerance Cometh: IgG4 After Multiple-mRNA Doses
IgG4 surges, and lab-apparent T Cell targeting of infected cells declines following a 3rd Dose of the Pfizer/BioNTech Covid vaccine, in a new study from Bavaria.
Spike-overload finally seems to be showing a concrete effect in the repeat-injected: B Cells in two separate cohorts were found to be self-switching to IgG4 class antibodies, associated with tolerance and anti-inflammatory response, after the 3rd dose.
Commenter Jim H did me the splendid favor of directing my attention to a new pre-print about IgG4. It’s a game-changer.1

So, let’s review this incredible and totally unforeseeable2 discovery.
Cohort 1: Here Comes… IgG4?
29 Healthcare workers were sampled at multiple time points. Spike-binding IgG4 begins to rise some time before 3rd Dose, increases again afterward.
So, conversion to IgG4 is already underway before the 3rd Dose, though half of donors are still under the limit of detection. Then the 3rd Dose, and a big surge. (It is not surprising that once B Cells for IgG4 antibodies are out there, another dose of mRNA for spike will cause expansion of IgG4 antibody.)
This is a totally bonkers thing for an anti-spike-protein B Cell to decide to do, and reflects B Cell over-exposure to spike, which reflects super-excess production of spike by the Pfizer/BioNTech mRNA code (although there are no unvaxxed controls, the study finds no IgG4 after AstraZeneca injection).
There is no reason to predict that this would be “good” in an antiviral response (in my opinion; but note there are theories to the opposite3).
About IgG4
In the “Y” of an IgG antibody, the base comes in four flavors.
While the two arms above the base will determine binding to target molecules (i.e. “antigens”), the base determines interactions with other immune proteins and cells. In effect, the four IgG subclasses are four separate “commands” (leading to sets of “effects”) that can be issued to the immune system for how to deal with whatever the arms have attached to.
A B Cell arrives at the arms-and-base design for its specific receptor/antibody in part by punching holes in its own DNA until the result codes for the thing’s arms and base. (The same way the previous sentence can be turned into, “A B C at the holes in its codes for the base,” and many other strings; then imagine that “holes” and “codes” can be mutated to bind to the target antigen as B Cells vie to create the best match.)
It does this when T Cells send it signal molecules saying “keep changing the thing’s arms and base, dumb-dumb;” but it can also do this when it encounters immune-exogenous molecules (like a bacterial carbohydrate) in absence of any such direction.
To review:
When IgG antibodies attach to their target, their subclass (base structure) represents the “command” that is sent to other immune cells and proteins.
Class-switching (changing the base of the antibody) is achieved by the B Cell knocking its own genes around. Distinctly, the tolerance-promoting IgG4 base requires knocking out all the other base genes. As in the sentence (emphasis added):
The relatively terminal position of the Cγ4 cassette may be one of the reasons why IgG4 responses tend to occur after repeated antigen exposure4
Pro-tip:
Once a B Cell has switched to IgG4, it cannot switch to any other IgG subclass, as the genes for all those other base designs have been discarded.5 All future clones of this B Cell will code for IgG4 receptor/antibody for the antigen in question.
IgG4 promotes tolerance, appears after long antigen exposure
To compare IgGs 1 and 4: IgG1 is the most abundant subclass, and is usually what is being referred to by “IgG” (whereas the other flavors are specialty/niche). It commands the immune system to absorb or destroy the stuff it is attached to, which is good in an antiviral response.
IgG4 is the rarest subclass. It does not command for destruction. To quote a 2014 review (emphasis added):
Allergens are often good inducers of IgG1 and IgG4, in addition to IgE. IgG4 antibodies are often formed following repeated or long-term exposure to antigen in a non-infectious setting and may become the dominant subclass. Examples are long-term bee keepers and allergic individuals that underwent immune therapy (8, 29–31). In immunotherapy, relief of symptoms appears to correlate with IgG4 induction. Switching to IgG4 may be modulated by IL10, linking this subclass downregulation of immune responses or tolerance induction6
“Wearing out” the immune response in this way is believed to contribute to the development of tolerance against tumors.7
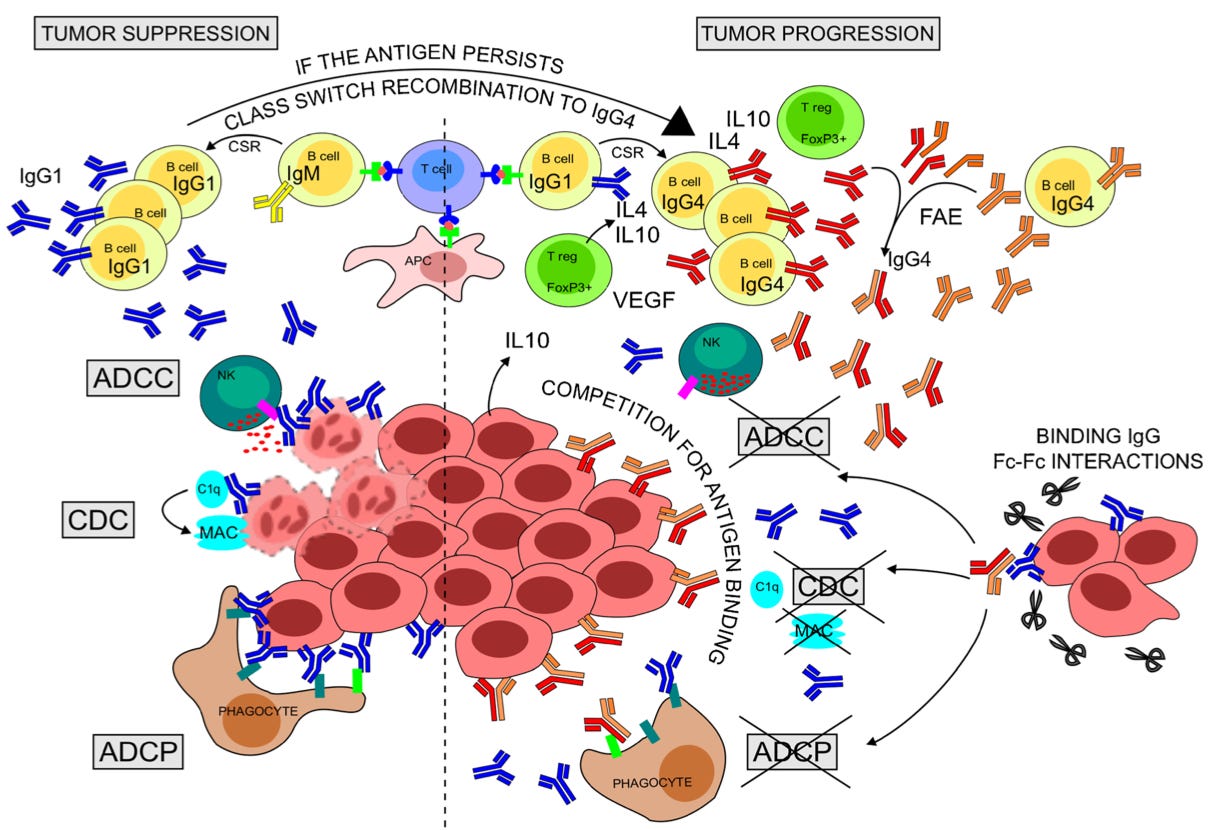
From the caption:
Rapid production of anti-tumor IgG1 can eliminate antigen-expressing tumor cells [yay!] through CDC, ADCC, and ADCP [a bunch of terms for destroying bad cells].
Chronic antigen persistence along with a Th2-biased cytokine milieu (IL-4, IL-10, VEGF) expressed by resident Tregs and tumor cells can support sequential CSR of B cells to IgG4. [B Cells can also convert to IgG4 independently of this regulation.]
IgG4 might thus be more affinity matured than clonally related IgG1 and may compete with IgG1 for binding tumor antigens [oh no!].
Crescioli, et ak. further offer a theory that immune suppressing IgG4 will have higher affinity than immune boosting IgG1 due to having emerged from B Cells that spent more time editing their bindy-arm design (“variable region”). They will be like strong-gripped bouncers shoving the rest of the immune system out of the tumor’s door.
We can therefor imagine that all those mRNA-transfected cells expressing spike after the 3rd or 4th dose or beyond, should any of them decide to undergo metaplasia as a result of the metabolic stimulation of the mRNA (as I suggested in October), will already have a leg up in terms of evading the immune response.
And now back to our new study.
IgG4 Switching and T Cells
Irrgang, et al. (“our new study,” at the time this post was written) take a look at the question of whether Th2 Helper T Cells are mediating conversion to IgG4, in accordance with the present understanding of how class-switching operates. They wind up producing an ambivalent conclusion.
Another preprint has appeared which reports the result of an accidental experiment more suited to answering the question.8
In Valk, et al. an ongoing study into Covid vaccine responses among patients receiving immunosuppressants for immune mediated inflammatory disease was used to look for any effect on eventual IgG4 conversion (found, as with Irrgang, et al. to be underway a while after the 2nd dose, and to intensify after the 3rd dose, in unmedicated controls).
They found a compellingly extreme reduction in patients receiving inhibitors for either the IL-4/IL-13 receptor or the cytokine TNF. A third drug had no such effect.
IL-4 is a cytokine associated with proliferation of Th2 and T follicular helper (Tfh) cells during immune responses, and is implicated in T helper mediation of IgG4 class-switching. That blocking IL-4/IL-13 blunts IgG4 conversion demonstrates, rather convincingly, that T Cells are mediating post-mRNA IgG4 class switching.
The authors point out that persistent Tfh cells have been observed after mRNA vaccination. In the referenced study,9 SARS-CoV-2 spike-sensitive Tfh cells are plentiful in both lymph nodes and blood after BNT162b2 (Pfizer) vaccination, and these persist for the entire observation window (up to 200 days after the first dose). This study involved a small cohort of young adults, but results were consistent.
Persistence of Tfh cells in the blood was nonetheless featured by a decline from initial highs; whereas lymph node germinal center Tfh populations remained high, suggesting prolonged development and maturation of the anti-spike immune response after the second dose, before any additional doses. This is once again in line with the model I have proposed for IgG4 conversion — as much more the result of the long-term effect of the first doses, rather than a consequence of the booster. Of course, boosters can only hurt further — but if the 2nd dose’s effects on IgG4 take time to show up, then there is no reason to assume that even 1 dose alone could not also cause IgG4.10
Back to Valk, et al., the (substantial, but less-so) efficacy of TNF inhibition is less obviously consistent with what is known about IgG4 conversion as TNF is a more generic cytokine employed by a gamut of immune cell types. Suffice it to say that our understanding of the immune system is incomplete.
IgG4 Switching Is Specific to mRNA Covid Vaccine
And so the authors checked whether donors who had received AstraZeneca’s chimp adenovirus chimera were also converting to IgG4. Instead, they found that vector + mRNA induced a partial conversion, compared to double-mRNA-injection which more strongly induced IgG4 switching.
While results in other subclasses are a bit interesting, they seem likely to be driven by the small sample size.
More importantly, the double-“ChAd”-dosed will have to substitute, in our evaluation, for a true unvaxxed cohort. It clearly seems as if the Pfizer/BioNTech shot is what is driving the B Cell conversion to IgG4.
The authors further explore whether super-dosed tetanus vaccine recipients show any sign of IgG4; or whether it can be observed in response to lifetime natural exposure to Respiratory Syncytial Virus:
In my opinion, they do find that repeat injection with the Tetanus toxoid leads to IgG4 conversion - but not to anything like the extent observed after 3 mRNA Covid vaccines.
Conclusion: It is not normal to make IgG4 when repeat encounter with a virus is spaced out over a lifetime, but injection-prompted antigen exposure promotes this response, and mRNA vaccines accelerate this effect.
IgG4 Switching Reproduced in 2nd mRNA Cohort
The authors next checked if IgG4 was increasing in an unrelated donor cohort. These donors were also sampled at four points (as shown above). Only two time points are shown for this group; my best guess is that “post 2nd” means 14 days, due to the high level of IgG1. Again, a clear rise in IgG4 after the 3rd:

Thus, the major finding of the 1st mRNA cohort has been replicated: Spike-binding B Cells are already switching to IgG4 some time after the 2nd injection, and these expand after the 3rd.
Cohort 2, per an offered citation, appears to include both general population and healthcare workers.11 Overall, this is a robust demonstration that the observed rise in IgG4 in Cohort 1 is not an artifact of some environmental exposure. But it would still be helpful to see if these results are consistent in a non-HCW population, to understand if tolerance is partly being driven by contact with other mRNA recipients due to some sort of “critical mass” effect as I have speculated previously.12
IgG4 Switching is limited to spike-binding B Cells
The authors further eliminated any question that their donors were simply converting to IgG4 left and right. In 4 donors examined from the 2nd Cohort, the 4 subclass was only elevated in (a small sample of) IgG B Cells binding to spike, not to the rest of the IgG clade:
IgG4 Dampens Apparent Cellular Immunity
The authors checked if IgG4 seems to be affecting T Cell replies to the spike protein. First they confirmed that recombinant anti-spike IgG4 antibodies had such an effect (compared to other subclasses with the same variable region). Then, if Cohort 2 sera after the 3rd Dose inhibited T Cell replies vs. after the 2nd Dose. It did:
These results would suggest that in vivo, spike protein will attract less notice from T Cells due to decoration with IgG4 antibodies. Naturally, this could lead to increased symptomatic infection, prolonged or worsened symptoms, and higher rates of post-Paxlovid rebound.13
Post-mRNA Infection Increases IgG4 Less than Boosting
Lastly, the authors checked how IgG4 replies to infection, either after the 2nd or 3rd dose. Particularly with 2nd-dose + infection, they find “smaller” increases in IgG4 compared to third-dosing alone.
Bearing in mind that additional doses of spike must lead to expansion of existing IgG4-class B Cells, it is difficult to decipher whether these short-term results speak for the long term; but overall, it seems like infection still leads to less additional spike-exposure (and B Cell tolerance) than injection.
A further insight offered here is that the Week 1 “baseline” for the 2nd-dosed is lower at fewer days post-vaccine (“dpv”). This appears to support that the over-sensitization and tolerance observed in the 1st cohort emerges gradually. For post-3rd-dose baselines, the highest levels appear in the early days, followed by a tapering off, reflecting that some of the increase is a short-term response to acute spike exposure.
What now?
Ultimately, the lack of further sampling time-points after the 3rd Dose renders perilous any guesses about what will happen next.
Probably, the Wuhan-spike-directed B Cells of triple-dosed mRNA Covid vaccine recipients will continue to convert to the tolerance-promoting IgG4 subclass the more they are exposed to spike.
However, novel B Cell responses after infection with the Omicron siblings (see “B Cells Anonymous”) may add new IgG1 B Cells to the pool.
On the other hand, the already-injected may just keep adding fuel to the fire with more injections, promoting more and more B Cell conversion to IgG4, in which case there is nothing to be done.
Related:
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Irrgang, P. et al. “Class switch towards non-inflammatory IgG isotypes after repeated SARS-CoV-2 mRNA vaccination.” medrxiv.org
Or not.

Regarding tolerance specifically, Igor Chudov has also recently raised concerns:
With the tolerance element being gifted by the brilliant Markael Luterra (who wrote the same ecosophia post highlighted by Chudov), I also addressed the possibility last September, in my “Forever Spike” theory.
The “severe efficacy is tolerance” theory forwarded by “Radagast,” which I do not find compelling at all. I want my immune system to react to a virus spike protein with an antiviral response, not as if it is part of me.
Vidarsson, G. Dekkers, G. Rispens, T. (2014.) “IgG Subclasses and Allotypes: From Structure to Effector Functions.” Front Immunol. 2014; 5: 520.
The initial version of this sentence described IgG4 B Cells as not being able to switch to any other class. As IgA2 is downstream of the gene for IgG4, and the literature is a mess, I am not able to confirm that a switch is not possible.
(Vidarsson, G. Dekkers, G. Rispens, T. 2014.)
Crescioli, S. et al. (2016.) “IgG4 Characteristics and Functions in Cancer Immunity.” Current Allergy and Asthma Reports volume 16, Article number: 7 (2016)
Valk, AM. et al. “Suppressed IgG4 class switching in dupilumab- and TNF inhibitor-treated patients after repeated SARS-CoV-2 mRNA vaccination.” medrxiv.org
The conflict-of-interests statement on this preprint is quite substantial, with two tangential references to Pfizer. Given that it elucidates aspects of an unexpected problem with the mRNA vaccines, it serves as an example of why I don’t fret and scold over on-paper conflicts of interests as a generic issue. It’s hardly as hazardous to the truth in scientific research as ordinary human confirmation bias.
Mudd PA. et al. “SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans.” Cell. 2022 Feb 17;185(4):603-613.e15
It seems obvious that the mRNA vaccines both deliver too much (nucleotide-modified) mRNA, calling into question the “need” for a second dose to begin with. Though this isn’t necessarily what drives the long duration of germinal center responses, it’s worth noting that other differences vs. the vector vaccines wouldn’t seemingly last very long, e.g. the pro-inflammatory nature of the LNPs. At all events, mRNA overkill is a reason to suspect that just one dose may be enough to induce IgG4.
Amount of mRNA was intentionally maximized, at least in the development of Pfizer (I have done less reading for Moderna’s process, but obviously they went beyond Pfizer in amount of mRNA anyway). It was not set to meet any “just enough” standard.
To summarize Pfizer’s Clinical Overview (accessible by searching “clinical” at https://phmpt.org/pfizer-16-plus-documents/), BNT162b2 was trialed at different doses (1, 3, 10, 20, 30μg) with 12 young and 12 older subjects, in parallel with BNT162b1 which only had a young cohort; as well, 60 and 100μg formulations were tested for BNT162b1, but due to higher side-effects after the first dose, these were not re-used nor trialed in BNT162b2.
The top-line statement of the rationale for choosing 30μg was that it had “robust immunogenicity in both younger and older Phase 1 participants” (section 2.5.1.2.5); but as no standard for “robust” is ever formulated in advance or retrospect, this statement simply means that 30μg made a bit more antibodies then the others — which isn’t any different from simply saying “bigger dose best, ung ung.” As such the Phase 1 immunogenicity comparison with lower doses was irrelevant; the highest that could not obviously cause more side-effects was chosen, and no attempt was made to set a standard for “just enough protection.”
This is revealed by a few quotes in section 2.5.4.4, e.g. “For younger BNT162b2 recipients at the 10 to 30 μg dose levels, Day 85 neutralizing GMTs ranged from 1.3- to 1.9-fold that of [post-infection antibodies.]” — Therefore, the difference between 10 and 30 wasn’t that great, and a post-infection benchmark suggested that 10 is as good as natural immunity.
Also, “For BNT162b2, by 7 days after Dose 2 (Day 29) GMTs had increased substantially in younger participants who received doses of ≥3 μg and in older participants who received 20 μg.” — From the perspective of a one-size-fits-all standard — “robust immunogenicity in both younger and older” — 20 seemingly should have been the final choice; but for the young, 3. That is, had there been anything like a real option to choose something less than 30, which there was not.
Finally, “On Day 43 (3 weeks after Dose 2 of BNT162b2), neutralizing GMTs in the younger groups decreased at the 3, 20 and 30 μg levels. Thereafter, neutralizing GMTs remained stable up to Day 85…” — Therefore, 30 μg wasn’t chosen because of any substantial difference in durability of immune response.
This leaves only one detail that can justify the decision to maximize the dosage — a higher boost after Dose 2 — but this applies to BNT162b1.
Tenbusch, M. et al. “Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2.” Lancet Infect Dis. 2021 Sep; 21(9): 1212–1213.
See “The Hot Spot.”
See “Unfinished Business.”

















Brian, this is frankly an awesome post that deserves great publicity. I will see what I can do.
"totally unforeseeable"
I would not call it tolerance. IgG4 leads to desensitization. Ongoing exposure to spike can lead to IgG4 related disease (IgG4-RD). IgG4 induction is the second stage. It is most likely preceded by IgE induction. Injecting any antigen will induce IgE. I warned against it. I also wanted them to measure IgG1,2,3,4. Finally someone has done it.
https://publons.com/wos-op/publon/34397456/#review-9360603