RashoCRON
Musings on a "sibling lineage"
The first thought I had, after I had absorbed a general impression of BA.2, Omicron’s sibling strain, was, I’m so glad I didn’t medicate my infection. Nothing arrested the replication of Omicron inside my cells except my immune system; the memory immune response generated from my encounter is thus as robust as it could possibly be. If there is a “sibling” strain waiting to re-challenge my immune system in a few weeks, I think I will be ready.
My second thought was, “I want my brain fog back.”
BA.2 has broken the formerly stable link between arbitrary variant nomenclature and real-life infection outcomes.
No person infected with SARS-CoV-2 makes nine trillion perfectly identical copies. The reality is more Elvis impersonators filling a convention hall, than Elvis in the dressing room. The defining distortion of sequencing is that it will always Revive the King - synthesize a coherent ideal viral genome from a hoard of flawed imitations. That we can still create from these consensus-snapshots a chronology of Elvis Himself - showing that this version of the King preceded this version - is a miracle of order perpetually emerging from chaos.
The miracle has been going on since Wuhan. Even back then, the eventual mutations that came to define first D614G, and later the Alpha, Beta, and Delta variants, might have already been present in the first-sequenced samples. But sequencing, by recording a “consensus” genome, will only show the currently most common sequence, not the soon-to-be-ascendent stepchild scrubbing the floorboards.
One of the most revealing Mouse Serial Passage experiments in the “Mouse Party” research timeline is Zhang, et al.1 Here, a vintage Wuhan isolate is subjected to a single passage through the lungs of an aged mouse, “creating” a mouse-adaptive Q498H mutation out of thin air. Isn’t it at least likely that this mutation was already present in the Wuhan isolate? Residue 498 substitutions feature in five other mouse serial passage experiments; again, these might all have been already present in every isolate later used for these experiments, even though later (human-grown) variants didn’t feature the mutation.
What’s more important is that this might explain why the D614G mutation could be included in a strain grown from “pre-D614G” isolates: It was already in the wild, for those first few months of 2020, before it suddenly showed up in the GISAID database.
In an asexual genome, fitness-conferring mutations do not always rise straight to the top of the pile. “Clonal interference” ensures that the most common version of a gene enjoys the advantage in replication - including in the replication process of genetic sequencing. So-called evolutionary / genetic clocks that purport to show how quickly SARS-CoV-2 can evolve, are in fact only measuring how quickly it takes beneficial mutations to overcome this interference. This timeline is more fluid than the timeline behind true mutation (though that, too, may change, if SARS-CoV-2 is now infecting both mice and humans simultaneously).
Likewise, whichever of the Mouse Serial Passage experiments that might have resulted in “Omicron,” might have simultaneously cultured and unleashed a multitude of related, descendent clades, that are not sortable in reverse with evolutionary clocks. Consider, again, Montagutelli, X. et al., in which the authors alternated between four strains of lab mice while making their way to a total of 26 passages:
If BA.1 and 2 share 38 mutations, while BA.1 has another 20 and BA.2 another 27 unique signatures to their name, this need not reflect anything except that the crucible of serial passage weakened the normal effect of “clonal interference” in perpetuating a suboptimal consensus genome. This is the normal dynamic for an asexual genome that thrives in a new niche: Beneficial mutations rise to the top more quickly.
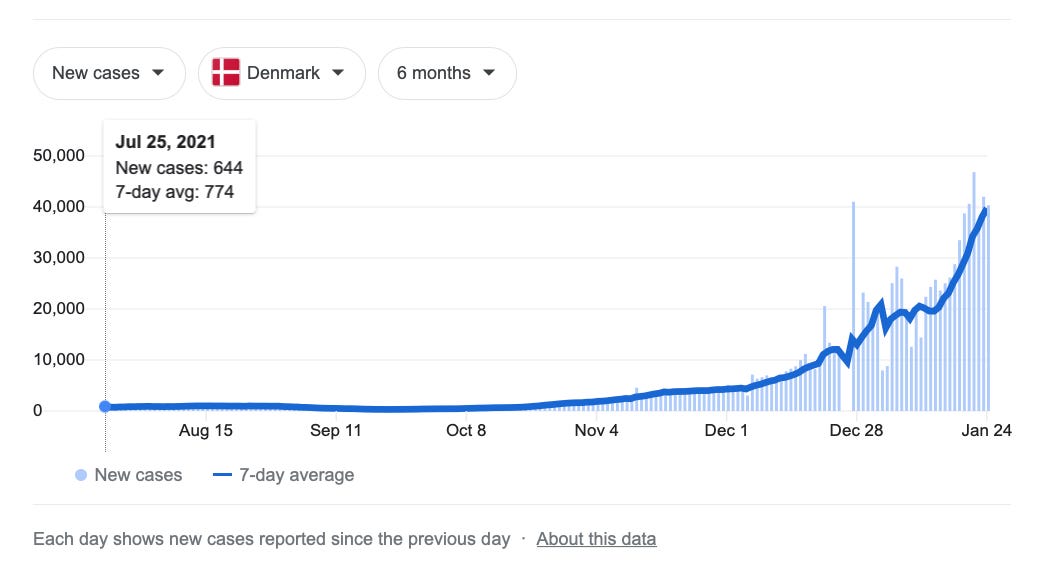
At all events, the fear is that BA.2 is a sort of pre-loaded Omicron reinfection. Just as soon as BA.1 encounters resistance from custom antibodies, the fear goes, BA.2 will keep the flame of infection burning. This is speculated to be what is driving continued rising cases in Denmark, absent any actual evidence demonstrating rampant “Omicron reinfections.” Instead, “Denmark cases = up” must be proof of BA.2’s influence, simply because BA.2 is increasingly prevalent in the country:
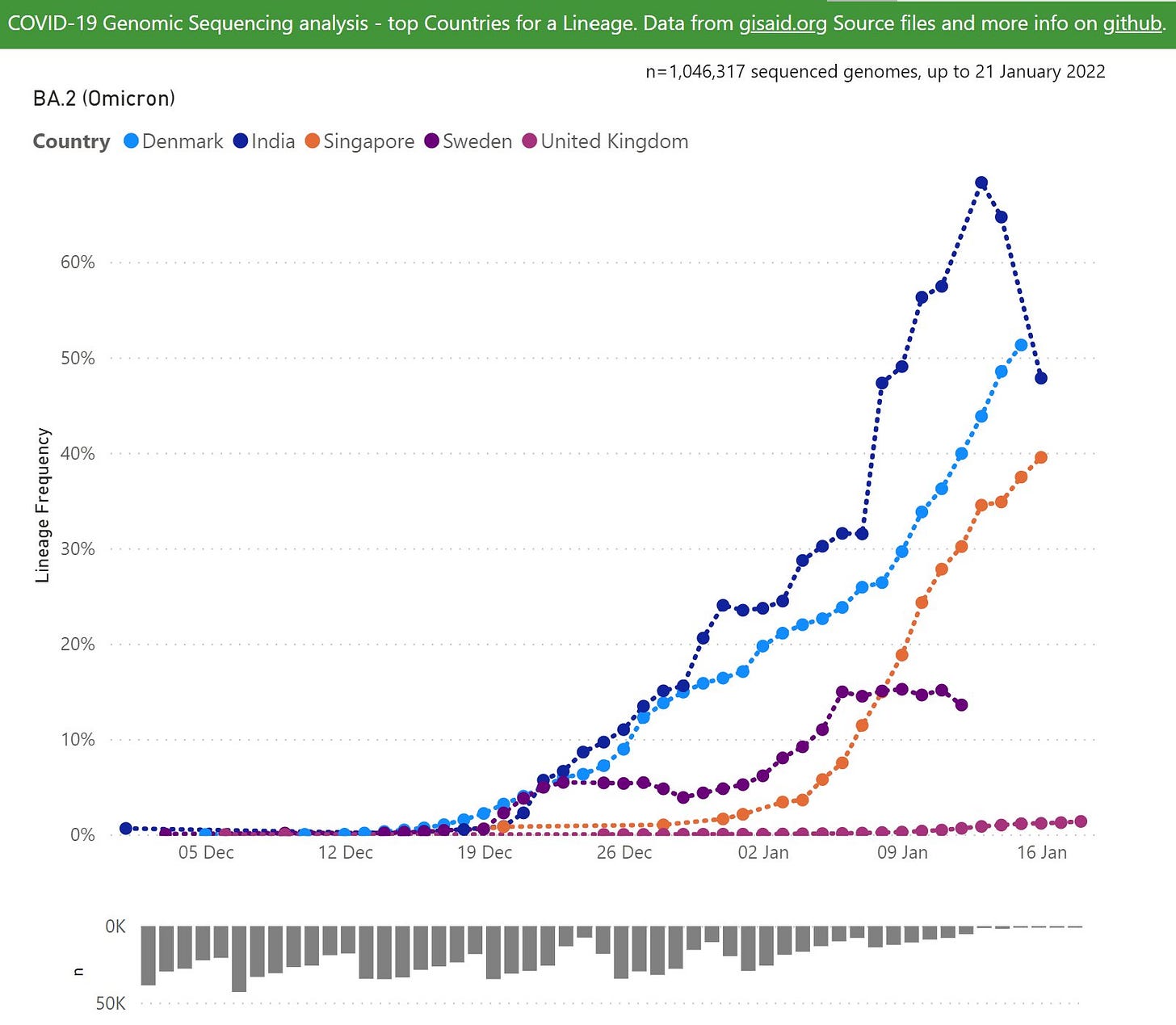
Well, maybe Denmark will prove to be prophetic, or maybe it won’t. There are lots of regions, especially in the US, where the Omicron wave already seems to be fizzling out. So when will BA.2 show its face there?
More to the point, it should be remembered why “Omicron” evaded prior immunity to begin with: It wasn’t just antibody evasion. It was a change in chemistry. BA.1 Omicron replicates in the upper respiratory tract. It further does not require TMPRSS2 in order to bind with ACE2, giving it higher affinity for infection in the young.
So, is BA.2 “Omicron,” or something new (or old)? If it is “Omicron,” then post-Omicron immunity will include a hoard of new resident T Cells in the upper respiratory tract, likely suppressing the sibling’s attempted attack.
But if not, then BA.2 may possess the more complicated molecular requirements for cellular entry that limited earlier versions of SARS-CoV-2 - so that it is more prone to asymptomatic infection, but less able to infect or transmit in the young.
The reader will note I haven’t mentioned anything about antibody recognition, even thought BA.2 is 12+7 mutations removed from BA.1 “Omicron.” So what?
What matters is the tropism conferred by the unique mutations to the spike. BA.2 must either retread the same ground as BA.1 (in the upper respiratory tract, where cellular immunity will be on high alert for those who recover from Omicron) or must attempt to launch infection in more hostile realms (where Natural Killer cells will easily snuff out infection for those with functional innate immunity).
Even if BA.2 rewrites the spike protein from scratch, there is a limit to how many ways the story of a “respiratory infection” can be told. For SARS-CoV-2, that limit may have already been reached.
See “Mouse Party.”




I'd like to see a chart comparing "cases" with tests. I expect they'll correlate pretty well. We've been mass producing tests, and hypochondriacs are lining up in the snow to get tested. Not surprising that would also increase asymptomatic cases. Every time I hear of someone with infection, they're uncomfortable til they get zpack or ivermectin, then they're fine. Cases are a scam.
Around Thanksgiving, all three members of my household had mild symptoms and tested positive on antigen tests multiple times. Then, just before the New Year, we succumb to a second round of positive antigen tests, but rougher symptoms. I believe my child was the one who brought the first round to our family from school and that the low viral load kept the adults' symptoms very mild. The second bout, a high viral load after one adult was in a car and recording studio with a double vaxxed who had just returned from holiday travel to Mexico. Each of us was hit with fever and body aches to start. I had the worst symptoms to follow- extreme fatigue, heart palpitations, altered taste and smell and cold sweats. Surely, after being hit twice with different strains (presumably, no sequencing done) - we are done, right? Please say yes. I am done being fucked with by humans playing god.