Mouse Party
Omicron as the product of human attempts to exploit the human-mouse ACE2 niche.
Early 2020 isolates of SARS-CoV-2 have been used in mice “gain-of-function” experiments throughout the last two years, potentially accounting for Omicron’s origins.
Note: A full-fledged theory for the origin of the Omicron siblings is now available in “Omicron Origins.”
c. 2019: The Creation of the Niche
Upon the release of the iPhone, fourteen years ago on this day1, thousands of niches for novel activities and physical structures were simultaneously created - some sparse, some rich, and few which would turn out to have anything to do with making phone calls. Most of the activities and physical structures corresponding to these niches would not appear right away, but would take years to be brought into existence, first awaiting the discovery of the corresponding strings of symbols which code for their creation.
The strings of symbols that successfully occupied these niches were, at length, discovered, refined, and published. They were plugged into the iPhones themselves, or into design schematics which translated into instruction-sets fed into circuits in factory robots, or API documents, youtube tutorials, coffee-stained-notebooks, etc. Others already existed. Immediately after the iPhone’s release, the ratio of measurements and lists of materials which corresponded to an “iPod Docking Station” filled a newly vibrant and expanded niche. Four years later the Lightning connector, devised to transfer data more quickly2, banished the Docking Station niche from existence. The strings of symbols which corresponded to its creation thereafter ceased to replicate. They went extinct.
In 2010, the invention of Instagram brought into existence an entire new biome for information codes - though these, too, took still more years to discover (aside from the now-obsolete faux-film filters which initially nourished the app to success). By 2019, these codes were driving millions of impressions in youtube tutorials, were domesticated in the walled gardens of knowledge in digital marketing agencies, were still being spontaneously created in the experimental recipes of ramen shops and ice cream parlors, and were being discovered throughout the pre-existing, relatively ancient genetic codes of nature3 - in the same year, a potentially artificial genetic code emerged which rendered all of these other codes obsolete at once.4
Which brings us to the “revelations” of the paper by Cameroni, E. et al., “Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift.”5 This paper suggests that upon the release of SARS-CoV-2 into the world in 2019 (if not earlier6), the niche currently filled by Omicron - a spike protein Receptor Binding Domain that binds to both human and mouse cells - was simultaneously created, and has been waiting all along for the corresponding code to fill it.
The arrival of a variant which possesses adequate affinity for both mouse and human ACE2 receptor implies that Omicron may be occupying an entirely different, and likely much richer, niche than that occupied by SARS-CoV-2 thus far. It can be spread to humans by mice, and vice-versa. This alone could account for the unprecedented rates of spread observable in The Campus of the Media, a.k.a. New York City, and elsewhere (including, potentially, Australia, which seems to be censored in the Google results at the moment7).
On the other hand, the higher affinity for tissue in the upper respiratory tract suggested by other experiments may also contribute to defining the niche. Additionally, it’s possible that previous variants have already been exploiting domesticated-adjacent animal niches without our awareness - though it is hard to imagine a better cross-vector than the mouse (since pets reside and travel with their already infectious owners anyway). It may also be that this niche is too volatile, or does not help sustain the virus in less urban areas, implying that extinction may be Omicron’s eventual fate. Nothing appears conclusive; I merely want to set the possibility on the table: SARS-CoV-2 has found its ideal niche.
The methods used by Cameroni, et al. to produce the chart above are highly synthetic, and in real life there may be other factors which are relevant to efficient infection of mice than just “binding-ness” to ACE2. But the inference that Omicron is “mouse-optimized” is strongly reinforced by the paper by Wei, C. et al. appearing on the same day, “Evidence for a mouse origin of the SARS-CoV-2 Omicron variant.”8
Here, the authors account for the disparate rate of mutations in the spike protein by comparing the motifs of these variations with those favored by the virus itself and by different hosts. These motifs arise out of the electrochemical conditions that operate while the virus is forming either the positive or negative copies of its genome within a cell: Some “improvisations” will be more favored than others. Change the virus, or change the cell, and the corresponding mutational motif changes in kind. Omicron possesses some classic SARS-CoV-2 hallmarks: “Transitions” are more favored than “transversions;” C→U is favored over G→A. But it lacks a signature created by high presence of Reactive Oxygen Species which can make “G” less available during viral replication in human cells: higher G→U than C→A. Notably, the authors find that these motifs have resumed in the weeks following Omicron’s emergence back into the human population.
This disfavors the (already so-2000-and-late) suggestion that Omicron emerged from a chronic infection in an immunocompromised human. With this strong evidence that Omicron has spent its pre-outbreak mutational journey off the (human) reservation in hand, the authors use magic formulas to map the spike protein mutations in Omicron against a few non-mice suspects in the coronavirus host lineup (mouse comes out on top9), and finally compare it to, well, previous lab-created versions of SARS-CoV-2 designed to efficiently infect mice.
So… it turns out… some humans have been trying to create a mouse-optimized version of SARS-CoV-2 for over a year.
And even worse, some humans already understood that such a version would be effective at infecting both mice and humans.
May, 2020(?): The Discovery of the Niche
My current intuition is that Omicron is a descendent of a lab-created, mouse-optimized mutant SARS-CoV-2 in early 2020. The papers listed by Wei, et al. indicate at least nine such mutant viruses were created in a lab (two papers refer to second versions of earlier mutants), some in 2020, and some more recently but using 2020-era live virus samples as the template.
The study that seemingly preceded all others was centered on “MA,” a recombinant mutant virus created in early 2020 at UNC under the auspices of Ralph Baric, and possibly the viral prometheus which delivered the knowledge of the mouse-human niche to mankind.11
MA was SARS-CoV-2 with an artificially-modified spike reverse-engineered from the mouse ACE2 receptor.
We used reverse genetics to remodel the interaction between S and mACE2 resulting in a recombinant virus (SARS-CoV-2 MA) that could utilize mACE2 for entry.12
It went quite well: MA could naturally infect mice, without the requirement that the mice were genetically altered to over-express human ACE2 beforehand.
Eventually, MA was refined via serial passage into MA10, with results posted online in September:13
Ostensibly, the purpose of creating MA was to foster a more “lifelike” infection in mice than that which occurs when mice are genetically altered to express human ACE2. Thanks to mutant MA, non-mutant mice could be used as a “model” for infection in humans, opening up a wonderland of mouse-based research for comorbidity dynamics, therapeutics, and prospective vaccines.
The creation of MA was inappropriately classified as “not dual-use,” as in not potentially being weaponizable. MA and MA10 were deemed to require BioSafety Level 3 precautions only, and potentially made available for other researchers.
By current US review standards, SARS-CoV-2 MA was not considered dual use research of concern, which is limited to a subset of pathogens. Prior to the start of studies, appropriate discussion and documentation of proposed experiments and protocols were reviewed and approvals obtained by institutional and external review. We recommend that SARS-CoV-2 MA and its derivitives be maintained in a biosafety level 3 laboratory.14
This, despite the fact that the paper announcing the creation of MA admits discovery of cross-infection potential in humans.
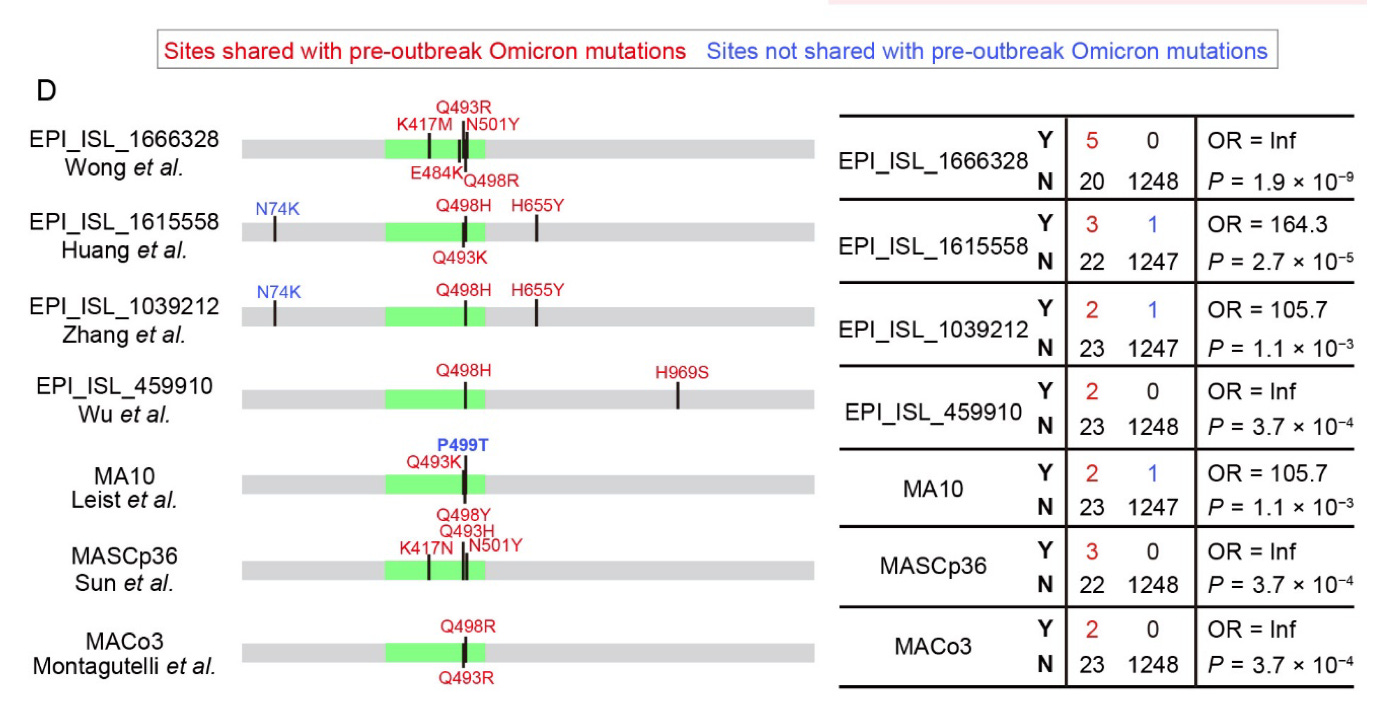
In a single, damningly comprehensive visualization, the authors demonstrate proof that their mutant virus retains likely affinity for human ACE2 in silico (computer simulation) and vitro:15
“A” displays the older viral gene sequences used to derive the mutant spike design in MA, including a mouse-optimized SARS-CoV-1 created via serial passage in Baric’s research in 2007.16 In “E,” the authors demonstrate that MA’s 498Y mutation retains function in a simulated interaction with human ACE2. In “G,” MA (the red dots) is demonstrated to grow almost as efficiently in Human Airway Epithelial cells as Wild Type SARS-CoV-2. In “H,” the authors provide an elegant visualization of MA’s cross-infectivity to both human and mouse ACE2.
When the full results for MA were published circa August 202017, the mouse-human niche became open-source knowledge. Anyone who wanted to could have cooked up an Omicron.
July, 2020 - Present: The Afterparty
If MA is not just the theoretical but in fact the physical ancestor for Omicron, this could account for the variant’s notorious genetic resemblance to circa-2020 SARS-CoV-2 strains. MA’s later development at UNC - the serial passages that led to MA10 - brought about a further mutation: 493K. Wei, et al. point out that 493K was responsible for enhancing binding to both mouse and human ACE2, whereas Omicron’s similar-but-different 493R only enhances binding for mice.
For example, Q493H and Q493K were also detected in the mouse-adapted SARS-CoV-2 at residue 493 of the spike protein, in addition to mutations observed in Omicron (Q493R). Different from the effects of Q493R, these two mutations increased the binding affinity toward both mouse and human ACE2 (Fig. S2C), indicating that SARS-CoV-2 could potentially evolve remarkably high diversity in its adaptation to ACE2 from various host species.
A portent of doom? Here, perhaps, we should apply a grain of salt to the results of the binding simulations: There may be other molecular interactions which make 493R more optimal at cross-infection between mice and humans in real life; or it may simply be that “not enhanced” is not important: 493R is adequate at binding with human ACE2. Additionally, even if Omicron becomes more infectious via discovery or reversion to 493H or K, that might not make it more severe.18
The distinction between Omicron’s 493R and the sequence published for MA10 - nor any MA mutations absent in Omicron that I may have left out - do not disprove inheritance. The serial passage infections at UNC likely produced a universe of mutations, a few thousand of which may have been viably transmitted from mouse to mouse in the lab, and any one of which could easily have escaped into the wild in 2020: One of these non-sequenced MA versions could, in fact, have featured 493R instead of MA10’s officially sequenced 493K.
All of these potential events could potentially apply to the other published mouse-optimized mutants (compiled in footnotes19), or to additional unpublished experiments. Multiple people may have solved the mouse-human niche “puzzle” independently.
No one appears to have been trying to stop any of them.
Related:
(it wasn’t on this day)
(defunct footnote)
(defunct footnote)
(defunct footnote)
Cameroni, E. et al. “Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift.” biorxiv.org
As provocatively suggested by “Ethical Skeptic.” I am a bit less convinced than originally by the latest update, but still find the overall premise a plausible account for the pre-existing immunity or semi-immunity of the China-Africa axis and Pacific regions (including California)
(defunct footnote)
Wei, C. et al. “Evidence for a mouse origin of the SARS-CoV-2 Omicron variant.” biorxiv.org
See Dinnon III, K. et al. “A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures.” (the creation of MA) Nature. 2020 Oct; 586(7830): 560–566.
and Leist, S. et al. “A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice.” (the creation of MA10) Cell. 2020 Nov 12; 183(4): 1070–1085.e12.
(Dinnon III, K. et al.)
(Leist, S. et al.)
(Dinnon III, K. et al.)
(Dinnon III, K. et al.)
See Roberts, A. et al. (2007.) “A Mouse-Adapted SARS-Coronavirus Causes Disease and Mortality in BALB/c Mice.” PLOS Pathogens. 2007 Jan;3(1):e5.
From my cursory skimming of the paper, there is no indication that SARS-CoV-1 MA15 was cross-tested for affinity to human ACE2. I haven’t yet reviewed the research corresponding to the other models used to reverse-engineer MA (including the imfamous “A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence”) as it is not immediately clear why they were relevant to mouse ACE2 binding. Nonetheless, the potential that the mouse-human niche was well-understood in advance of the creation of MA is responsible for the inclusion of a “(?)” in my dating of the discovery.
The May pre-print version of the paper, which does not seem to feature results of MA vs human ACE2, is archived at https://www.biorxiv.org/content/10.1101/2020.05.06.081497v1.full
As severe outcomes from SARS-CoV-2 infections, again, seem more to do with inflammatory reactions to other elements of the spike protein, which are apparently absent in Omicron. I do not profess to know the relevant prolines by name.
Dates correspond to earliest online post-date yet found. * are updates to previous listings. Sites shared with both BA.1 / BA.2 are in bold:
May, 2020, UNC, USA: “MA”
Dinnon III, K. et al. “A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures.”
(See “MA10,” below)
July, 2020, Beijing?, China: “MASCp6”
Gu, H. et al. “Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy.”
(See “MASCp36,” below)
August, 2020, Harbin, China: “HRB26M”
Wu, S. et al. “A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge.” and Wang, J. et al. “Mouse-adapted SARS-CoV-2 replicates efficiently in the upper and lower respiratory tract of BALB/c and C57BL/6J mice.”
Lab: Harbin Veterinary Research Institute (BS4)
Source: SARS-CoV-2/HRB26/human/2020/CHN (EPI_ISL_459909)
Mice: BALB/c
Passages: 14
Mutations: nsp8 A81T, S Q498H, del675-679, N969S
*September, 2020, UNC, USA: “MA10”
Leist, S. et al. “A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice.”
Lab: UNC Chapel Hill (BS3)
Source: cDNA SARS-CoV-2 genome (just lying around the house, naturally…) transfected into Vero E6 cells to produce virus, as best I can tell
Pro-mouse mutation: Q498Y/P499T via primer-directed cDNA edit, as best I can tell
Mice: BALB/c
Passages: 10
Positive for hACE2 binding: In Vitro and In Silico
Mutations: nsp4 T285I, nsp7 K2R, nsp8 E23R, S Q493K (Q498Y P499T were edits), orf6 F7S
*November, 2020, Beijing?, China, “MASCp36”
Sun, S. et al. “Characterization and structural basis of a lethal mouse-adapted SARS-CoV-2.”
Lab: ? (BS3)
Source: “IME-BJ05” (Isolated early 2020, Beijing)
Mice: BALB/c
Passages: 36
Mutations: nsp3 I1258V, nsp4 H470Y, nsp5 S301L, nsp6 A128V, nsp8 S8F, nsp11 P84S, S K417N, Q493H, N501Y, N R32C, D128Y
March, 2021, Huazhong (Wuhan), China: “LG”
Zhang, et al. “SARS-CoV-2 Rapidly Adapts in Aged BALB/c Mice and Induces Typical Pneumonia.”
Lab: Huazhong Agricultural University (BS3)
Source: Wild Type, from WIV (EPI_ISL_402124)
Pre-passages: Vero-E6 X 9 (EPI_ISL_1039208)
Mice: BALB/c (aged)
Passages: 1
Final: EPI_ISL_1039212
Notes: Perhaps pre-existing Q498H in source isolate “swarm”
April, 2021, Iowa City, USA: “SARS2-N501YMA30”
Wong, L. et al. “Eicosanoid signaling as a therapeutic target in middle-aged mice with severe COVID-19.”
Lab: University of Iowa (BSL3)
Source Virus: “WT-SARS-CoV-2 BAC” (Wuhan / Wild-Type with A20085G and G26840C marker mutations), from Sonja Zuniga and Luis Enjuanes (CNB-CSIC, Madrid, Spain)
Pro-mouse mutation: N501Y via PCR fragment→BAC-transfected E Coli recombination
Pre-passage: Vero E6 and Calu-3
Mice: BALB/c
Mouse passages: 30
Multiple strains isolated, only one preserved and named
Mutations: nsp4 T295I, nsp8 S76F, nsp9 T67A, S K417M, E484K, Q493R, Q498R, (N501Y was an edit)
May, 2021, Huazhong (Wuhan), China: “WBP-1”
Huang, K. et al. “Q493K and Q498H substitutions in Spike promote adaptation of SARS-CoV-2 in mice.”
Lab: Huazhong Agricultural University (BSL3)
Source: Wuhan-Hu-1, from WIV
Mice: BALB/c
Passages: 11
+Screening of isolates for most virulent strain
Final: EPI_ISL_1615558
Mutations:
Positive for hACE2 binding: In Vitro (Fig 3)
July, 2021, Paris, France: “MACo1 - MACo3”
Montagutelli, X. et al. “A mouse-adapted SARS-CoV-2 strain replicating in standard laboratory mice.”
Lab: Institut Pasteur (BSL3)
Source: BetaCoV/France/IDF0372/2020 (Isolated January 2020, France)
Mice: CC071 alone and with CC001 and C57BL/6, then BALB/c
Passages, mutations:
15 (MACo1)
16 (MACo2)
27 (MACo3)
New (not referenced by Wei, C. et al.):
December, 2021: Brisbane, Australia “MA1 - MA5” (no relation to MA/MA10)
Yan, K. et al. “Passage of SARS-CoV-2 in cells expressing human and mouse ACE2 selects for mouse-adapted and ACE2-independent viruses.” (hat-tip to Markael Luterra)



















Excellent, thanks!
So if mice can now spread Omicron, there is even less point in locking down or vaccinating -- good luck eliminating all the mice. And of course the virus will continue to evolve in mice, and cross back to people.
The "pandemic" cannot be solved, except by everyone acquiring immunity.
I would like to add a few mammals to your footnote. Over here in the Western US we had a epidemic of Rabbit Hemorrhage Fever (which is attributed to SARS-CoV1, believe it or not). Affected rabbits act sickly, then suddenly bleed out through their noses (sound familiar?) and die. A friend lost 80% of his rabbits to this. My does (female) rabbits were both pregnant at the same time. One delivered a single live kit and a number of malformed, dead fetuses; the other doe delivered a malformed kit that died at birth and a bunch of clumps of bloody flesh. Can't say if they would have died from thrombocytopenia as well, as I figured they were done and turned them into dog food. Shortly after that, all my ewes aborted. Two other friends with goats saw their nannies either abort or deliver malformed, unviable kids. Correlation isn't causation but....