What the FDA’s same-day statements justifying their authorization of 4th doses reveals about the current state of affairs.
And so, here we are.

In the media call posted to youtube, Peter Marks emergency-provides an account of the rationale for today’s emergency-adjustment of the Emergency Use Authorization to Emergency-Authorize the 4th Emergency-Use of the mRNA Covid Emergency-Vaccines in the elderly and immunocompromised, as soon as 4 Emergency-Months after the 3rd Dose (consult Section II.4916a of your Emergency Almanac if confused).
The gist of this rationale, which doesn’t become clear until Marks’ faltering reply to the fifth and sixth callers’ questions, is that there is virtually no data available to support the FDA’s decision, but also “no data”1 to dispute it - and so they just decided to make the adjustment for kicks.
In his opening remarks, Marks announces the FDA decision via abstract and polished platitudes, essentially offering no direct justification for authorizing the 4th dose to begin with. The authorization is merely described, and the implication of that authorization - that something has gone “wrong” with the protection of the previous “booster” - is aggressively downplayed. The 3rd doses are OK, the 3rd doses are doing better than OK, in fact the 3rd doses are beyond spectacular. So Marks’ statement doesn’t even seem designed to promote 4th dosing to begin with.
Indeed, when the 4th caller (11:50) asks Marks to specify the urgency attached to this decision - who should get the 4th dose, and who should wait? - Marks portrays the authorization as only an invitation for already-boosted individuals over 50 to “seriously consider” shooting up another time. Again, the elephant in the room - are the 3rd-dosed in some sort of unprotected state? - has gone undiscussed, and Marks hasn’t really substantiated any of the text of the FDA’s announcement at all.
And that’s when things go south.
15:20 - Why a 4-month interval? Answer: “Directly from the data from Israel.”
It is only when Marks is asked to justify the 4 month interval that he first stumbles, and begins to actually advocate for today’s announced policy change - thereby revealing how flimsy the rationale for that change is.
Marks asserts that “the paper from Bar-On” was instrumental in the 4-month decision, presumably accounting for the otherwise yet-unreferenced, top-line assertion in the FDA press release:
Emerging evidence suggests that a second booster dose of an mRNA COVID-19 vaccine improves protection against severe COVID-19
However, this isn’t even a rational justification for the specific question. As Marks says, Israel authorized 4th doses with an “at least 4 months” interval from the 3rd dose; but in practice the authorization made elderly residents eligible 5 months after the authorization of the 3rd dose. To recycle my Chrome-translated screenshot of the dashboard from late summer (the buttons are sticky; this is actually a 3-month view):
So, most boosted elderly Israelis probably received their 3rd dose in August.
January is 5 months after August; coincidently the same interval as the FDA’s 3rd dose recommendation before today. So the Israel 4th-dose decision can’t do anything to justify modifying the established FDA precedent from 5 to 4 months. And the detail that Bar-On, et al. used the same “cutoff” doesn’t change the fact that most of the subjects in their study were likely 5 months out from the 3rd dose. So as he elaborates his description of the rationale, Marks is just spewing raw, FDA Grade A hot air:
The 4 months comes directly from the data from Israel. So they, they, uh, the data that comes when you, when you look, um, uh, at, at, at the data that we were able to review, and you’ll see this in, uh, the paper from Bar-On, that is, uh, referenced in our, um, uh review memo, um, they [Israel], they [Israel], they [Israel] instituted their booster campaign in, uh, in people who had been vaccinated at least 4 months previously. They [Bar-On, et al.] did their data analysis using that. And so, the data that we have, um, that, uh, indicates, uh, the, uh, potential benefit against hospitalization and death, comes with an interval of 4 months [no it doesn’t]. Um, and so that, that’s how we came to that 4 month interval.
Given the actual timing of 5 months between doses, the limited evidence for severe efficacy of a 4th dose vs the 3rd dose in Bar-On, et al.’s paper, which we will discuss below, doesn’t actually support a 4-month window. So the conservative assumption, which is certainly not contradicted by Marks’ caught-off-guard tone, is that said paper’s results have nothing to do with the interval.
A more natural rationale for a 4 month interval wouldn’t refer to severe efficacy at all: Instead, it would be based on the waning of infection efficacy, which has been pegged to 3 to 4 months for both the original 2-dose course and for boosters. But if the FDA is merely trying to keep elderly Americans juiced-up on high anti-spike antibodies around the clock (with unknown health and immune impacts) just to recapture the dream of durable infection efficacy, Marks obviously has no intention of admitting it.
And so, what of that alleged improvement in severe efficacy?
From Israel, with Inscrutability
Bar-On, et al., as it happens, have become the principle intermediary between the Israel Ministry of Health and the public at large, when it comes to discrete post-booster outcomes for individuals in their database. They issued a previous view on infection and severe outcome rates immediately after the 3rd dose in August, and now have published one for the 4th dose in January.2
I’ve previously applied a “light touch” to the former paper,3 but now that the FDA is citing their latter work as justification for an amendment to the EUA, I think it is appropriate to take off the gloves. The Bar-On data does nothing to support a long term benefit from either booster, only a transient apparent benefit, or at the least a lack of negative efficacy.
It cannot be but thus: If their analysis of the 4th dose shows a short term benefit vs the 3rd dose, then their own, earlier 3rd dose analysis has in some manner been rebuked, which means that their 4th dose analysis cannot be trusted to hold up either. Neither analysis should be considered dispositive without reconciling whether the efficacy of the 4th dose is the product of a deterioration of the efficacy of the 3rd dose, or what.
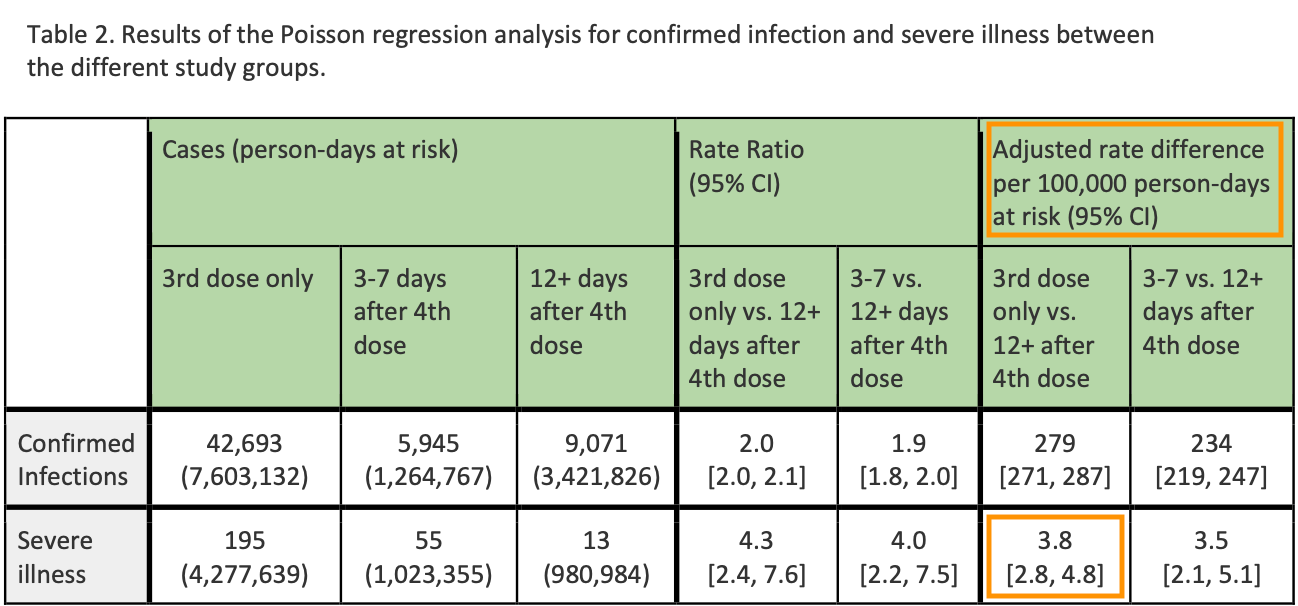
Either way, Marks’ off-balance rambling winds up asserting that the FDA deemed a 4-month “top-up” for the already-boosted essential based on a study that only follows post-4th dose severe outcomes for a single week.
Here, “12+ days” really just means 12-16 days (since the 12+day population is only at ~58% of the end-of-window size at the halfway point). For the “12+” but really just 12-16 days post-4th-dose group, severe outcomes were less frequent than for the 3rd only group. While “not currently infected user bias” and the reduction of the denominator for the 3rd-dose only might have partially driven this result, and while the biology suggests an improvement to severe outcomes in such a short time window shouldn’t even be possible, my back-of-the-envelope math suggests that the design isn’t too flawed here.4 But the issue is not the flaw, but the limitation.
It was just Days 12 - 16!
How can the FDA justify boosting on a 4-month schedule if they don’t know if it keeps offering a benefit over the next 3.5 months?!
19:20 Question unclear? Answer: Because we discounted side effects completely in this age group.
The next caller catches Marks off guard again, though it’s not even clear why. This time, the caller asks Marks to discuss if there is a “downside” for younger age groups, without specifying whether this refers to a would-be negative to 4th-dosing, or a would-be disadvantage to not 4th-dosing.
Bizarrely, Marks takes this as a prompt to discuss why harms from 4th-dosing for the over-50 group were not seriously evaluated in making today’s announced decision:
First of all people over the age of 50, the risk associated with an additional booster is very low. Um, the risk of myocarditis in this range from the vaccine, um, is, is really, uh, considered to be minimal. The side effect profile that was, uh, looked at, um, in, uh, a million individuals who had received additional doses, um, in Israel, looked very favorable, uh, in this age range.
Here, nothing would seem to substantiate Marks’ claim other than the text of the FDA press release itself, which refers to proprietary Ministry of Health data (emphasis added):
A summary of safety surveillance data provided to the FDA by the Ministry of Health of Israel on the administration of approximately 700,000 fourth (second booster) doses of the Pfizer-BioNTech COVID-19 Vaccine given at least 4 months after the third dose in adults 18 years of age and older (approximately 600,000 of whom were 60 years of age or older) revealed no new safety concerns [so what? were the old ones not enough?].5
Although I will, in an upcoming post, be discussing why post-1st-dose Covid vaccine recipients may be partly protected from the harms directly caused by the spike protein during subsequent injections, is an apparent “very favorable” side effect profile gleaned from a surveillance “summary” really good enough to give the FDA a pass on the 4th booster announcement? Especially if that summary is merely not worse than the already unacceptable results of the domestic surveillance signals collected for the first three doses in VAERS? I find the language heartless and robotic.
Marks’ invocation of the Big M is particularly telling: “The risk of myocarditis in this range from the vaccine, um, is, is really, uh, considered to be minimal.”
In other words, it’s not like adverse events among the elderly have made it to mainstream media headlines, so why should the FDA even give them any thought?! An excellent point. Absent outside scrutiny, exactly why should the FDA have to justify not “seriously considering” such harms to begin with, before recommending that the elderly “seriously consider” a fourth dose? Does Marks even get paid for that?
What a confession of sheer, unbridled indifference.
Great, so the elderly do not appear to experience higher rates of myocarditis.6 Myocarditis is easily revealed by an array of tests. What about cognitive decline? What about autoimmune and inflammatory diseases, including worsening of diabetes? What about cancer? Who cares. Take your subsidized experimental 4th transfections, because the first 3 don’t work enough anymore, m’kay? After all, it might keep a whopping 28 out of every 100,000 of you out of the hospital for a brief, one-week period in the height of a wave, so why should the remaining 99,972 of you be worth lifting a finger to look into side-effects on our part?
19:20 Question unclear, Pt 2? Answer: But also, everything’s fine!
Immediately after having acknowledged that side effects for the elderly were essentially waived away as “so, like, boring!” the reason for Marks’ dismissal becomes clear: For whatever reason, the innocuous wording of the 6th caller’s question has led Marks to outdo the ambivalent wording of his opening summary.
Whereas the opening summary seeks to describe authorizing 4th Dosing while affirming the protection of the 3rd Dose, Marks - as if trying to disguise a minor stumble as an intentional burst into sprint - now seeks to protect the image of the 3rd dose by admitting that the 4th dose (because it wasn’t considered to have a risk) should not imply a benefit.
So, um, uh, it, in this age range [50 and above], very favorable side effect profile, and the evidence that, um, there is some waning of protection the serious outcomes or that that protection could be improved by giving, uh, an additional dose [which one? eh, who cares]. [Onto the non-elderly:] There’s reasonable evidence right now that there’s still, uh, in younger individuals, um, uh, the protection against, uh, against the serious outcomes of hospitalization and death, um, is being maintained, and although it may be dropping off a little bit, um, uh, it, it’s not dropping off in a, uh, a very significant way. So, um, the feeling was, right now, at this time, uh, to take this action for the, um, those most at risk and obviously we’ll continue to evaluate the data, and if at some point, uh, if because of what happens with the evolution of Covid-19, uh, in terms of, uh, another wave, or because we get additional data that says that the additional protection is beneficial, um, we would, uh, potentially modify this in the future.
And so, the FDA has emergency authorized 4th doses of an experimental, gene-based medicine for elderly Americans. And a key representative of that decision, when asked to explain the implications of the decision, lest said implications would lead to questions about the efficacy of the 3rd dose, launched immediately into stressing that the decision had no actual rationale at all.
Bar-On, Y. et al. “Protection by 4th dose of BNT162b2 against Omicron in Israel.” medrxiv.org
As in my defense of the 3rd-dose paper’s findings regarding post-injection infection rates in “NorCal Pregnancy Study, etc.”
In this window, infection risk exposure and severe outcome exposure have pretty even denominators for the 12+ day group (assuming a lag between infection and severe outcomes of a few days), while the 3rd-dose only group is disadvantaged by a ~1.3X risk to person-day ratio. Note that person-days function as the volume of colored swirls in any given window, but since severe outcomes are delayed, it is helpful to focus on what is going on in the middle of the overall “severe period” window as compared to a same-sized chunk of the preceding window:
This disadvantage might be a bit smaller, as well as more balanced, if a shorter lag is assumed. On the other hand, any denominator disadvantage for the 3rd Only group would be compounded by selection bias - the already-infected are not going to receive the 4th dose to begin with, and so “severe outcomes” for the 12+ day group are discounted to some extent by the lower rate of infection that immediately precedes receipt of the injection. These things together may account for the resulting 4X severe outcome rate among the 3rd dose only group vs the 12+ group, but I don’t want to press the argument too far.
The text “clearing” the Moderna product is even more galling:
The safety of Moderna COVID-19 Vaccine, when administered as a second booster dose, is informed by experience with the Pfizer-BioNTech COVID-19 Vaccine [so what? that is not adequate for a higher-dose mRNA product] and safety information reported from an independently conducted study in which the Moderna COVID-19 Vaccine was administered as a second booster dose to 120 participants 18 years of age and older who had received a two-dose primary series and a first booster dose of Pfizer-BioNTech COVID-19 Vaccine at least 4 months prior [in other words, the Moderna 4th dose was approved based on only 120 trial subjects]. No new safety concerns were reported during up to three weeks of follow up after the second booster dose [so what? real-life recipients will not be protected from harms emerging after that window just because they weren’t studied in advance].
Though, a “low risk bias” could result in up to a 4X rate of myocarditis going undetected in older Covid vaccine recipients, as modeled in “Technical Difficulties.”








Darkness has descended upon the globe, America has led the world into this madness. We will lead it out, or we will see the dark-triad of BigTech, BigMedia, and BigGov consolidate global control by war, famine, and manmade-disease. It ends here, or it doesn’t end.
Heh, it’s also neat that when/if “journalists” go to quote from this call, the rules for a “professional” transcriptionist would be to omit the “uhs” and duplicate words. His blathering mostly ranges from content-less to horrifying anyway, but I think your record more accurately conveys the “flavor” of this decision than in whatever drivel the various Times of the world will puke out tomorrow.