Part 1 of the Unglossed OAS Lit Review dealt with the early history of flu research. Part 2 will deal with modern attempts to “prove” OAS.
The following review is intended as another supplement: A superficial examination of the initial debate over “Original Antigenic Sin,” such as it was, following the 1953 paper (reviewed in a previous supplement); as well as of the 1957 flu vaccine campaign’s total refutation of OAS.
Again, readers only interested in the modern “evidence” should skip this installment.
Overall, this series is intended not as essential (or even desirable) knowledge, but as a resource to refer to any time the proponents of OAS insinuate that the evidence supporting the theory was all iron-clad from the start. It was anything but.
The Lost Years: Introduction
In a sense, the initial research on “OAS” can all be treated as anachronistic and obsolete. None of it is included in Yewdell and Santos’s summary table, “selected studies on the human recall antibody (Ab) responses following influenza virus exposure.”1 Several of the new methods developed during this time have fallen out of common use (whereas the methods discussed in Part 1 endure today).
Nor do these “lost years” offer a firm answer to what - in the modern literature - OAS should be taken to mean. As there is no supreme court in science, the intention of the early authors of this term is unimportant in this regard. It only matters how “OAS” is used today; which is to say, totally incoherently.
So one essentially has to treat the initial and modern investigations into OAS as two separate worlds, with a literature review only able to rationally accommodate one.
I have finally decided neither to skip over this era entirely (my original intention), nor try to bring it to life in pristine detail. It is a decaying corpse; the following examination will take a twig to it here and there.
Itinerary:
Opening Shot
OAS claimed.
In 1953, Thomas Francis, Jr. and two underlings at the University of Michigan Department of Epidemiology (of which Francis was the founding chairman) published the observations and statements which formed the basis for the myth of “Original Antigenic Sin.”2 Emphasis added:
The results of this study also suggest that during the initial infections with influenza viruses, which occur predominantly in childhood, the major antigens of the prevailing strains have a unique effect upon the antibody-forming mechanisms which persists throughout life and largely determines the character of the future antibody with which this cohort of the population will respond when subsequently exposed to related strains of virus.
These observations were critically flawed as donors were pooled together and actual flu exposure histories were unknown, as previously reviewed.3 Yet note that even in this initial formulation, before the catchy slogan has been invoked, the language is sufficiently legalistic and vague as to allow for multiple interpretations and redefinitions: How is “character” defined? Does “cohort” only refer to responses a group, or individually? And does “related” strains mean distinct subgroups (within A) or not? The authors of the sentence can wait for an opponent to critique it before offering an answer to these questions.
If, for example, we take “character” to mean “strain-specificity of resulting immunity,” “cohort” to refer to groups as a whole, and “related” to mean a new sub-group within the A group, the statement above could be tested by the 1957 flu vaccine - which was issued and trialed in advance of the arrival of a new subgroup. Individuals injected with an egg-cultured new flu strain - one “related” to the ones previously in circulation - either developed protection against infection with the new virus (whereas individuals simultaneously injected with older strains did not), or not. Which was the case, will be reviewed below.
The Ann Arbor school, however, would not the year before have considered this a test of “OAS” as they came to define it. They would contend that this is an insufficient standard. They would define “character” (strain-specificity) not in terms of “development of observed immunity where it had not been before,” but “failure of post-exposure serum to resist cross-reaction with prior strains in artificial lab absorption tests that we ourselves conduct.”
Idiotic.
Besides which, their own study refuted.
The Battle of Ann Arbor, Pt. 1: Charge of the Absorption Brigade, 1956
OAS defined and refuted.
The first major effort to define-prove OAS comes in 1956, when the phrase still has yet to appear in print (though we can imagine Francis was already using it in casual correspondence):4
In this, the Ann Arbor school uses a novel tool - antibody absorption - to defend the theory hastily shot-off in 1953, by requiring refutation of the theory to jump through their newly invented hoop.
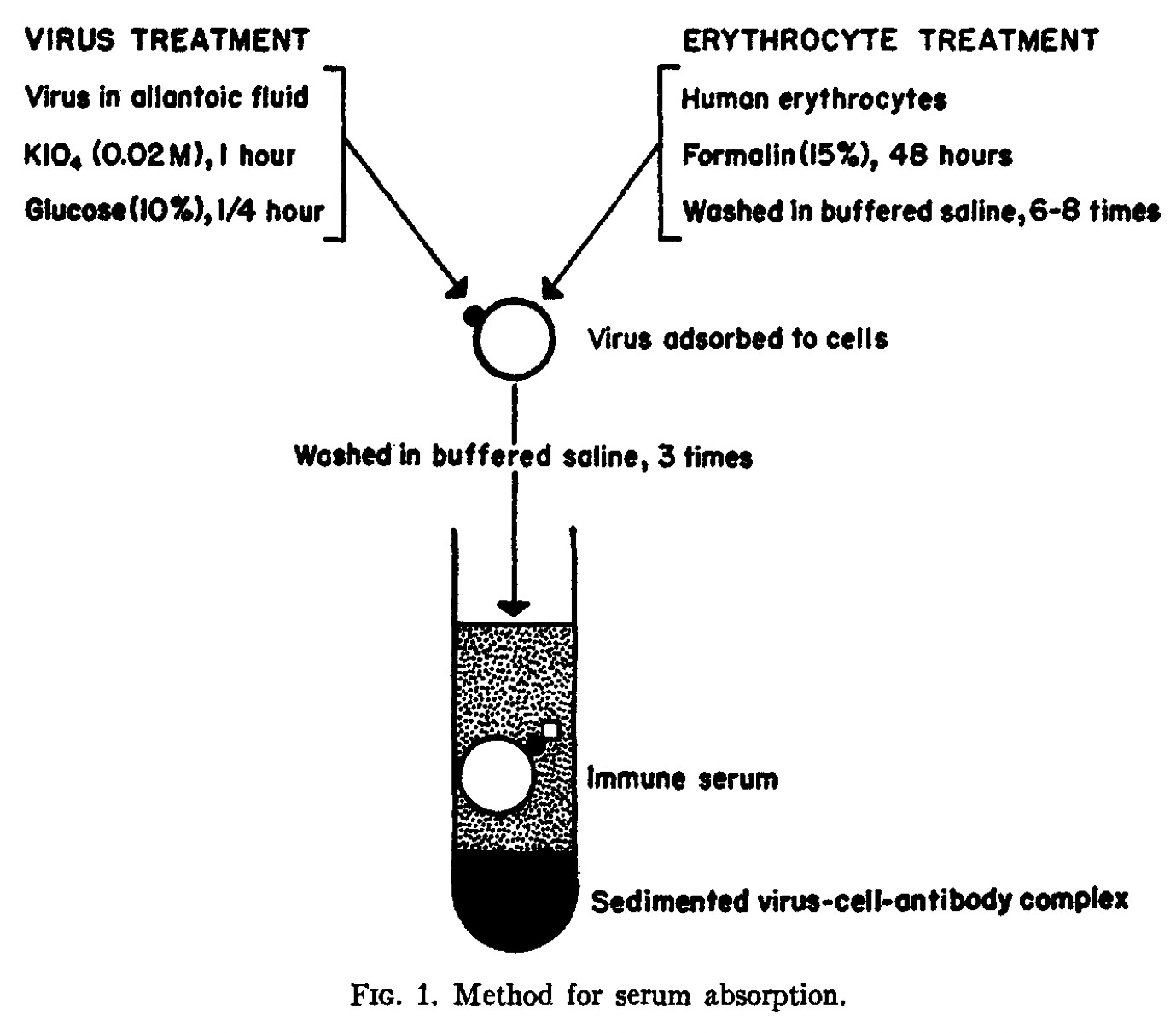
The process is visualized in a previous paper:
“Immune serum,” in the context of flu, contains antibodies that may be shown to react (protect against infection or red blood cell glomming) to multiple strains of flu at different strengths. Francis at this time was enamored with the way results for a given “immune serum” could be altered by screening it with given strains of flu. In his view, for example, if your blood showed reactivity with both PR8 and FM1, but screening with PR8 removed activity for both, you clearly only have antibodies for PR8. Setting aside all the myriad questions about accuracy, which the Ann Arbor school was scrupulous regarding, we can see how Francis found this an excellent tool to defend how the “character” of later immune responses was “largely determined” by early responses.
In the above scheme, had hawk not absorbed owl, or had owl absorbed hawk, then it would be false that the “character” of the anti-owl response was “largely determined” by the anti-hawk response. However, that did not occur, in this idealized example. Naturally, the next step would be to run the exposures in reverse on a different subject, and see if the statement “R1 largely determines R2” winds up being false that time (ideally, not), so that (ideally) you know that it is the order of exposure, not something about the different strains themselves, that is driving which strain determines the response.
What Francis and co. would thus boldly contend, is that they have demonstrated that even though the hawk-exposed subject can appear to develop an immune response against owl, those are really just cross-reactive hawk antibodies in disguise. By a modern understanding, this would imply that B Cell clones from the first response that happened to cross-react with owl expanded; but no new clones for owl were created (as has in fact been observed for post-Covid-vaccine infections with BA.15).
Again, we can set aside questions of accuracy or whether this is merely measuring amount of antibody vs. specificity (if owl could actually remove hawk at a higher dose), for now.
And so that was the approach taken for the ferret experiments summarized in the 1956 paper:

This is included as another idealized demonstration of what we “should” see in the human portion of the paper.
As a category, I do not intend to include animal “proofs” of OAS in this review, as OAS alleges to comment on a natural phenomenon, but the conditions of a lab are easy to manipulate in favor of producing an unnatural result. Moreover, the flaws in this model are obvious: First, as these are all closely-related strains, it is less interesting and certainly not alarming that a ferret should merely reuse and refine immune response from the first exposure - as there will be sufficient related antigens to defend against the sibling strains (in other words, the ferret is already immune). Second, we now know affinity maturation to continue long after apparent recovery, including in animal models, so comparison between a months-later immune response vs. a two-weeks later immune response may exclude authentically novel anti-Weiss antibodies that would appear later.
And so now that we have been introduced to Francis’s absorption game, how does OAS fare when human subjects are examined?
It is refuted.
Human donors are once again pooled, and grouped by age. In this case, age 4-10 (mental institution), 17-26 (military), and 30+ (mental institution) groupings of forced test-subjects are compared, which in 1956 results in three first-exposure “teams” of FM1 (“A Prime”), PR8 (“A” classic), and Swine (1918) flu subgroups. (Hopefully?6)
These go on to be given different vaccines among these three representative strains; but before the vaccines, an absorption test already defies OAS. While Team Swine loses all antibodies against PR8 and FM1 if their (pooled) blood is screened by Swine, Team PR8 still has FM1 antibodies after screening by PR8 (Fig 17). This means their existing FM1 antibodies are not “secretly” PR8 antibodies.
Things go worse after vaccination with the three different strains. All cases where the first-exposure strain cannot absorb antibodies against later strains are in violet ovals. Red and fuchsia rectangles , meanwhile, are for cases where later viruses can absorb “imprinting” strains, implying that the observer should stick to considering cross-immunity as a normal thing for related viruses that offers no great insight into the “character” of immune responses to begin with.
For the Adults set (Team Swine), some variance can be expected due to the inclusion of subjects born in the mid-20’s, when anti-Swine antibodies become rare. As for the Recruits, there is at least a small chance that the youngest individuals in that group missed out on the 1943/44 flu season, making them secret “FM1s.” Failure to include raw data leaves us unable to assess that possibility. Technically, the “largely determines” argument would insist that it is implausible or impossible for a single person to lack a determinate strain - one that can absorb all others. But these are pooled results.
With that caveat acknowledged, my highlights cover the lost “points” where OAS “should” have scored but did not. Overall, the Team absorbent strain offers 4 (Children and Recruits) to 6 (Adults) points for successful non-Team removal each round, and the non-Team absorbent strains offer 6 points for Team non-removal (2 of which are essentially giveaways, for Team non-removal after Team vaccine).
OAS’s score in the Francis Absorption-Dome
vs. Team FM1 (Children): 9/10
vs. Team PR8 (Recruits): 3/10
vs. Team Swine (Adults): 7/12
Total: 19/32
(needed: 32)
Among non-Children: 10/22
(needed: 22)
Result: Loss
OAS refuted.
Requiring a perfect score might seem unfair. But put differently, refutation of OAS successfully jumps through the new “antibody absorption” hoop 13 times out of 32. This is more than random error should allow, especially considering that these are still pooled results (recycling the low resolution of the 1953 paper)! Additionally, the results for Children should arguably not be counted since OAS is more concerned with predicting what the immune system does after childhood (“refutation” scores 12 of 22 points).
Lastly, note the orange wobbles. These measure the strength of immune response to the new strain (orange) vs. the “backboost” to the green strain (in the nil. results, before the post-vaccine samples have any crazy absorption tests performed). Here, we see the very original observation by Francis and Salk in the FM1 vaccine trial (in which old antibodies were higher than new ones) is only reproduced in 1 out of 6 cases. Once again the Recruits (Team PR8) offer a particularly strong counter-example here, with a 4-fold lesser PR8 response after FM1 vaccination.
What is Francis and Co.’s reaction to these findings? To re-display the results in the most confusing manner possible,8 stall the reader by droning on endlessly, and finally somehow conclude they nailed it.9
In view of this evidence there can now be little doubt as to the marked persistent influence an antigenic experience with influenza virus has upon the antibody-forming mechanisms of the virgin host.
For “imprinting” strains to fail to clear antibodies to new strains in 5 out of 10 cases (in Recruits and Adults) is sufficient to enable a “little doubt.” It is clear, contra the conclusions offered by the authors, that the novel antibody responses to later influenza A subgroups are just that.
Pacific Theatre: Asian Flu Vaccine, 1957
OAS refuted IRL.
The arrival of “A Prime” in 1947 had caught Francis and the rest of the flu vaccine apparatus by surprise, and forever dashed hopes that the “A” group of influenza was a serotypically static, homogenous entity. As a result, the flu vaccine apparatus was given a more formal structure, centralized under the WHO.
The antigenic shift resulting in the “Asian flu” of autumn, 1957, was caught in advance; successfully recovered the Spring before in Singapore; and promptly shipped to Hilleman at Walter Reed - allowing the theoretical rush-deployment of a vaccine before the virus hit the world stage, and putting the WHO flu machine to its first test.
The limitations against creating and evaluating a vaccine against this new strain were nonetheless great - but they were all of a logistical, not biological nature: China was not part of the new surveillance network, delaying discovery until early 1957 (the virus appears to have emerged the year before). The public health bureaucracy of the Eisenhower administration, burned by the Cutter incident 3 years before, was slow-footed in recommending or supporting production of a vaccine based on the new flu - resulting in a chaotic, controversy-strung rollout once summer outbreaks and media hype prompted spurts of increased demand.10 Lastly, the virus arrived in full force weeks earlier than expected, in October.
Nonetheless, the rushed injections against this novel A influenza appeared to range between 40 to 66% effective in preventing illness, in trials in the UK or America. Humans far and wide, despite being “sinned” with immunity to previously circulating groups/strains, had mounted an immune response tailored to a “subsequent” strain. The idea that they wouldn’t have done so is ridiculous.
Among adult RAMC and RAF volunteers in the UK - with all their presumed, “persistent” antigen exposure in childhood as well as myriad previous military vaccinations - injection with a two dose course of the new Asian flu vaccine induced protection even though the virus arrived while the second doses were being administered.11
The Asian vaccine was estimated to cause a three-fold reduction—that is, 66% protection—in the number of influenza cases as compared with the numbers in the [influenza-B-vaccine receiving] control group occurring nine days after [the second dose] and thereafter. Within the first eight days after [the second dose; and so, before additional seroconversion could take place] no differences were found [echoing 1943, when Francis and Salk’s injection appeared to work despite being delivered mid-wave]. The polyvalent influenza A vaccine [comprised of previously circulating strains] behaved just like the control influenza B vaccine, so it can be concluded that the stimulus of the up-to-date Asian antigen was necessary for protection. Recent published accounts of trials of Asian vaccines in the USA have given figures of effectiveness in the range of 40-67%.
The Asian flu vaccine lowered rates of observed illness (whereas an injection with familiar A strains, which would have led to generic recall of prior influenza A immunity, did not).
And so, thanks to this real-life experiment, OAS was refuted - a mere 4 years after it was proposed.
Grading on a Curve
Obviously, Francis’s contemporary definition of the “character” of these immune responses wouldn’t refer to something so quaint as protection from illness, but to a complicated array of post-absorption measurements. However, his own results for WS, PR8, and FM1 vaccines the year before demonstrate that the Asian flu vaccine “immune response” would likely pass this test.
On the other hand, if we reverted to a consideration of a “cohort” as individuals within a cohort, as opposed to as a group, this would open up the possibility that OAS was not refuted by these trials. Perhaps universal protection would have been observed if not for prior memory immunity against related strains, so that the new immune response was “hit or miss” because of the old immune response.
Of course, this embodies a circular argument, in which high immunity to older strains is proof that flu vaccines would work perfectly otherwise which is proof that high immunity to older strains is why flu vaccines don’t work perfectly. Indeed, this is speculated in another trial which used generation of antibodies as the endpoint, finding that 9 out of 25 subjects failed to meet benchmarks for the new Asian flu (but responded universally to an injection with older strains).12
And yet the results observed for the military trial in the UK were identical to a concurrent trial among public school boys:13
Even if these were all likely teenagers with prior exposure to A strains, it seems implausible that “OAS,” and not general limitations of injected vaccination against influenza, would cause the same failure rate across groups (adults vs. teenagers) with two different amounts of “sin.” As protection was similarly incomplete in the 1943 trial for Francis’s PR8-based vaccine14 - this taking place in a population which would have very little prior encounter with the long-gone 1918 strains - blaming prior immunity for the incomplete protection of the 1957 vaccine is an act of imagination, not induction.
It further defies OAS to explain why, during the 1957 Asian flu trials, antibody levels and observed protection against infection would rise after two doses. If the first dose was either defused (in terms of a novel immune response) by prior B Cell recognition or merely generated expansion of cross-reactive B Cell recognition, a second dose would not plausibly right the ship. Instead, it is more coherent to view the new immune response as developing despite prior B Cell recognition and expansion, via a distributed or “multi-channel” memory and novel B Cell response, as we now understand adaptive immunity to work (exactly as discussed in the prior supplement15). The first dose would have led to new B Cell lineages with affinity against Asian flu’s new physical chemistry (antigens); the second dose would have expanded those, leading to higher antibodies and resilience against symptomatic infections that otherwise would have developed due to encounter with the virus in the days afterward.
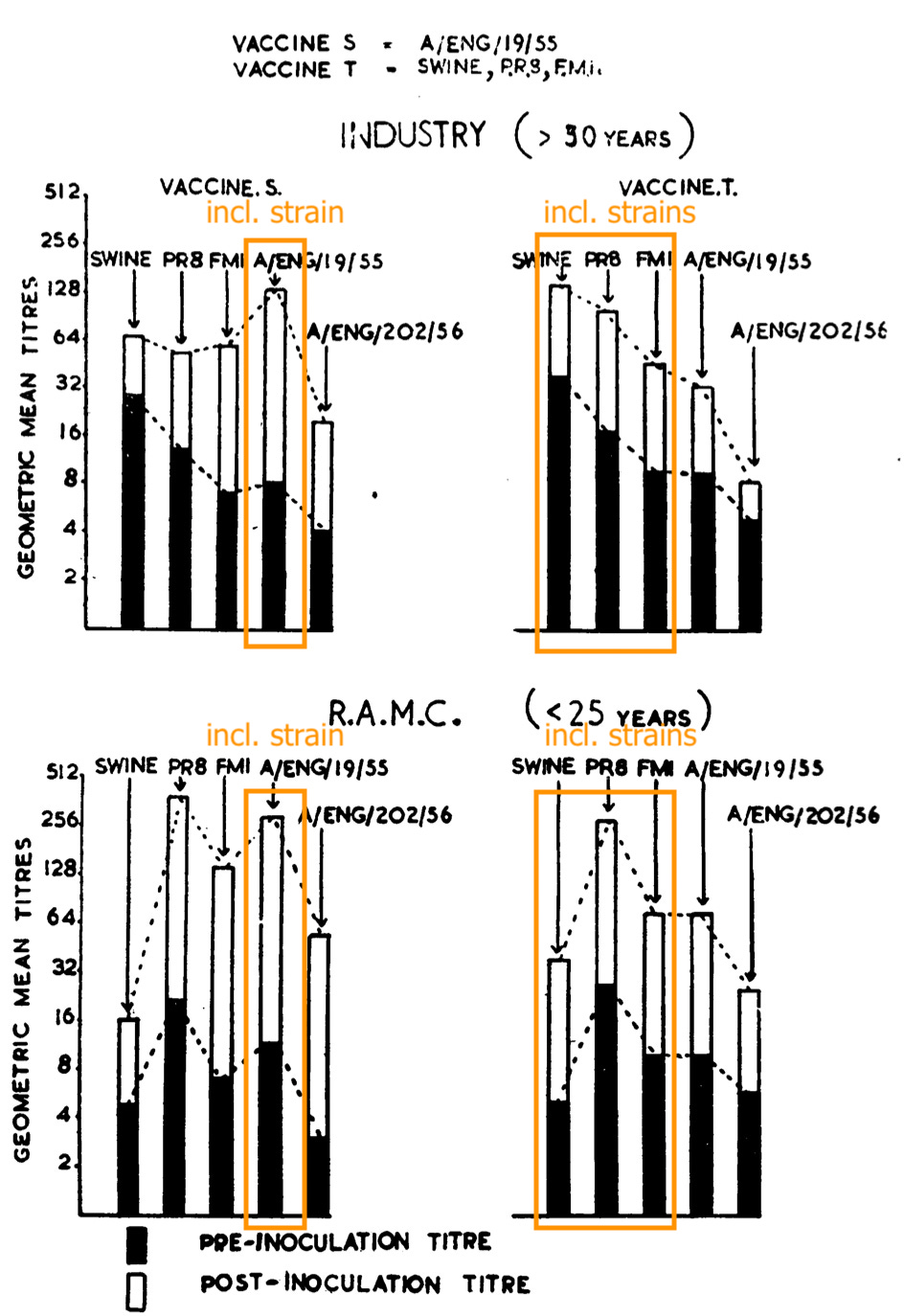
The UK Asian flu studies were prepared by the Commission for Influenza formed in collaboration with the WHO after the arrival of “A Prime” in 1947. Also of note is that the same commission refuted OAS in regard to within-subgroup vaccines the years before.16 Injection with the 1955 strain makes for donor blood that can be diluted more before it stops preventing the 1955 virus from causing blood-cell glomming than injection with previous strains:
Immune response against the ‘56 strain likewise ended up higher for the ‘55 vaccine than for the “classic hits” vaccine. On the other hand, the overall similarity of the circulating viruses to older versions seemingly explains why little difference was observed in call-out rates.
I have argued before, when claiming that OAS is not real, that what is meant by this claim is that no real-world demonstration of a relevance - a detrimental effect - to recall of old, cross-reactive immune responses has ever been demonstrated (besides for Dengue, which is ADE, not OAS). So it was in 1957: A rushed vaccine against a novel, “related” influenza A strain, when issued (barely) in advance of the next flu wave, had performed similarly well as the original PR8 vaccine did in 1943, despite prior immunity to non-Asian strains.
There is no real-life precedent for OAS.
The only “problems” remaining after 1957, from the perspective of a Public Health wonk who thinks the whole world should be repeat-injected with egg-grown virus juice just so a few of them stop calling in sick one time a year, were logistical and political. Could elected governments be mobilized more quickly? (Yes, in 1976.) Without backlash if the new flu doesn’t show up? (No.) And from a wholistic, long-term health perspective, should they, in the first place? (Assumed yes, for the wonk.) Logistical, political. Not biological.
That didn’t stop Francis and his school from continuing to attempt validation of the myth of OAS up to his death and after.
It’s a Trap (B.A.A. Pt. 2), 1966
OAS redefined.
With technical advances gaining pace in the 60’s, absorption was soon replaced by cleaner, more science-y ways to evaluate the specificity of the invisible things in blood that prevent flu strains from making red blood cells glom. These allowed for a somewhat clearer understanding of what flu “antigens” actually were, and that there were multiple of them, and that some appeared before the virus was assembled, some were shared while others were not, etc.
One of these new methods was photometry. Not much needs to be said about it except that the Ann Arbor school quickly co-published a demonstration that the pre-existing anti-Swine and anti-Asian flu antibodies possessed by those alive during the 1918 or 1889 flus, respectively, were indeed specific for those strains.17 For some reason, it doesn’t appear that they went on to offer any “proof” of the non-specificity of post-childhood flu immunity employing this new, reliable method. Seems like a missed opportunity to validate OAS (Francis-style) for good.
Instead, two years later, a new method of evaluating the equilibrium constants of antibodies was employed.18
But where this paper really breaks ground, is in redefining “OAS,” to now mean simply: Recall of antibodies to prior strains upon exposure to a new flu strain - in other words, cross-immunity - that is (ever? sometimes? always?) higher than the novel response.
Thus it has been known for over 10 yr now that humans vaccinated against influenza produce antibodies against the immunizing antigen, but produce antibodies of higher titer against the antigen that was their first childhood experience of influenza, even if that strain happened to be absent from the vaccine-- hence the name Original Antigenic Sin (1).
Where this is distinct from Francis and Co.’s original formulation (vague as it was) is that it no longer claims to “[persist] throughout life and largely determines the character of the future antibody.” It does not reprise the implications offered in the 1953 paper - that older generations weren’t making any new antibodies at all (an artifact of using pooled samples) - or in the 1956 paper - that any apparent new antibodies were really “secret” old antibodies.
While “Disquisitions” might deserve a review on its own grounds - it appears to perform a logical summersault, by claiming that antibodies against generated new strains are too good to be new - there will be plenty of examination of this “redefined” meaning of OAS using more modern methods in Part 2. It seems better to save ammo here. More noteworthy is that the first experimental report to use the phrase “Original Antigenic Sin” is also the first to retcon the original definition.
Also noteworthy, is that even the retconned statement - “humans vaccinated against influenza produce antibodies against the immunizing antigen, but produce antibodies of higher titer against the antigen that was their first childhood experience of influenza” - is at this point not an accurate representation of the published results. It would only be accurate if appended with the un-sexy modifier, “sometimes.”
To revisit the Ann Arbor school’s own results in 1956:
But the statement is not meant to be accurate, of course. It is akin to a vow of fealty, as in to the idea of OAS, regardless of the truth of the words within the vow - much like papers today must append “safe and effective” to the word “vaccine” lest their authors burst into a ball of flame.
The retcon is reinforced in the next prominent employment of the term, in 1974:19
The "original antigenic sin" phenomenon (OAS) 1 challenges the dogma of the specificity of the immunological memory: when there is sequential infection with two different but antigenically related strains of influenza A virus, the antibody stimulated by the second infection reacts more strongly with the primary virus than with the one actually eliciting the response.
The modern resurrection of the term will revive this trap, as well - where some papers refer to “OAS” as mere antibody recall; others, as a cosmically-mediated force which “exacerbates the severity of the current infection;” a “blind spot of the immune system” which causes “the redirection of responses to the ‘original Ag’ rather than to novel epitopes,”20 fully affirming the interpretation that OAS implies that no new antibodies can be formed.
And so almost from the beginning, “OAS” has been a distributed, motte-and-bailey rhetorical scam - where some will use it only to mean “recall antibodies for variant antigens” (i.e. cross-immunity), some “the end of human immunity as we know it,” and both sides will nonchalantly assert that the thing in question has been demonstrated in the literature over and over.
Make no mistake, the blame for this state of affairs lies with those who take post in the bailey - de St. Groth and Webster and their followers. By invoking “OAS” and subtracting Francis’s clear implication that true, “new” immunity is prevented by recall, they launder his original idea and defend it from the incredible flaws in the papers from 1953 and 1956, or from any modern test.21
For the reader who has followed this journey so far, what should stand out the most about the redefinition of “OAS” in 1966 and ‘74, is the paucity of “evidence” for the original definition there-to-date:
Pooled sera that obscures the immune response from actual post-childhood infections.
A grandiose, face-plant-failure to prove that novel, vaccine-induced antibody responses can reliably be absorbed by “imprinting” strain viruses.
And that’s it.
That’s the “evidence” for the true definition of OAS.
Rosebud, 1960
OAS dreamed.
This was as far as Salk would ever go in criticizing his mentor. He owed him far too much. But there were others in the younger generation who [in the 1940’s] viewed Francis as something of a fossil, a fussy administrator with declining scientific skills. “Have you seen Tommy’s paper in the recent Bacteriological Reviews?” a friend wrote to Salk. “A more garbled discussion I have never read…. And amazing it is that Francis is president of that organization.”22
While the Ann Arbor school’s output regarding OAS was limited after 1956, the overall pattern seems to have been to incorporate new techniques when doing so could advance the argument, and perhaps to ignore them when not. Overall, one may liken this quest to a man moving into a neighborhood and declaring, based on whatever he had gleaned from visual inspection on the drive to his house, that all of his new neighbors were “a busload of jerks” - and then producing volumes of supporting proof based on actual interactions in the years afterward (and obviously excluding all evidence to the contrary, like the Asian flu vaccine success).
“The evidence I based my conclusion on was faulty, but my conclusion was correct anyway!”
This pattern is an illusion. There is the revanchist effort to render “OAS” less extreme after 1960; and then there is Francis’s simultaneous re-affirmation of both the 1953 and 1956 formulations in 1960.23
First the pooled, 1953 results are treated as woke-up-like-this flawless (emphasis added):
The antibody of childhood is largely a response to the dominant antigen of the virus causing the first Type A influenza infection of the lifetime. As the group grows older and subsequent infections take place, antibodies to additional families of virus are acquired. But the striking feature is that the antibody which is first established continues to characterize that cohort of the population [when pooled] throughout its life [due to the dynamics of lower infection rates falsely depressing apparent response to newer strains when samples are pooled]. The antibody-forming mechanisms have been highly conditioned by the first stimulus, so that later infections with strains of the same type successively enhance the original antibody to maintain it at the highest level at all times in that age group [so what?]. The imprint established by the original virus infection [here disregarding numerous earlier acknowledgements that childhood features a greater quantity of infections] governs [there is no centralized “governing” of B Cells] the antibody response thereafter [no]. This [this, the 100% explicit governing of later antibody responses, not the mere recall of original strain antibodies] we have called the doctrine of original antigenic sin.
“This we have called”: Any reference to OAS that merely observes “imprinting” as being evident within later immune response, rather than governing it (in spite of all current knowledge of how memory immunity is not a centralized entity), is not comporting to Francis’s definition of the term.
Francis is not done. Next, the complete falsification of the working hypothesis in 1956 is simply edited out of the account:
The interpretation is well supported by other experimental data. Ferrets have been infected subsequently with viruses of the different families [wrong, it was different strains within the “A” classic family] with resultant antibodies to all three. When either the second or third virus was mixed with the final serum, only part of the antibody was neutralized, but treatment of serum with the initial virus removed antibody to all three viruses. Treatment of children’s serum with A-prime removed antibody to all Type A strains [there would not have been any pre-vaccine antibodies to pre-A-prime strains to remove]; the prototype A strain, PR8, removed all antibody from the serum of young adults [patently false; it left behind anti-FM1 antibody!]; swine virus removed all antibody from the serum of the middle aged.
No mention is then made of the complete failure to replicate these absorption results after vaccination. Instead, Francis touts pre-absorption antibody levels - while misrepresenting the results in all three age groups.
Vaccination studies have further demonstrated the influence of primary education [?]. Regardless of the strain of Type A used for vaccination, children responded most prominently with A-prime antibody [falsified by PR8 vaccine], young adults with A antibody [falsified by FM1 vaccine], and adults of 30 or more with antibody to swine virus [falsified by PR8 vaccine].”
Nothing can suffice to describe Francis’s characterization of his findings in 1953 and 56, save for outright mendacity.
Regardless, this seems to be a superfluous flight of dishonesty - Francis could have disowned his most extremist views on OAS, and still set up the audience for his concluding remarks. For this reason, we need to entertain an account for what OAS “meant” to Francis that treats the extremist, “governing” aspect as non-essential - a mere artifact of his dullness and arrogance, and the coddling, feather-touch treatment he habitually received from his underlings and colleagues.
If that was the case, then what does the conclusion of his lecture suggest was the thing that really obsessed him with OAS?
First, a check on what flu had been up to in these “lost years.”
The 1957 Asian flu virus, once recovered, turned out to be another time-capsule, like Shope’s Swine flu strain. But instead of being a marker for exposure to the 1918 virus, it could identify those who were alive during the 1889 flu. This reinforced Francis’s cyclically-minded theories of antigenic shift - he had proposed as early as 1945 that flu might rotate through a limited spectrum of available antigen motifs,24 implying that eradication of the virus was still possible. This was to be contrasted with Andrewes of Mill Hill’s school of thought, which favored a more intuitive (in evolutionary terms) assumption of virtually infinite phenotypic novelty.25
Is it in the light of this dream, that Francis’s obsession with “OAS” can be understood?
For context, the polio virus eluded the Rockefeller Institute’s efforts to define serotypes for decades, until John Enders (at the Children’s Hospital of Boston) discovered a tissue culture method that did not render the recovered agent too different from wild type. That was in 1949. The serotypes in circulation were then fixed at 3, and Salk’s polio injection was deemed ready for mass trial a mere 5 years later (with Francis helming the year-long analysis).26
Flu had been far easier to culture in a manner productive to serotyping; the problem instead was that the wild virus seemed to be taking years and years to show all its cards. “Polio-ing” flu, according to Francis’s theory, thus depended on charting the obscure landscape of gradually cycled motifs.
And so, Francis’s lecture proposed that a universal vaccine could be derived once this limited template was mapped, synthetically combined into a single injection, and given to children before they were “sinned” with a narrow-spectrum wild variant (which would forever allow them to only mount apparent new responses to future recycled antigens, not true-new responses).
Children represent the most susceptible members of the population and probably the most important material for the building of epidemics. The gaps in their immunity should be eliminated by providing early in life the antigenic stimuli to meet the known or anticipated recurrent strains. Natural exposure would then serve to enhance the broad immunity laid down by vaccination. It is our hope that such vaccines can be made from pools of chemically purified antigens—or even with strains experimentally devised. In this manner the original sin of infection could be replaced by an initial blessing of induced immunity.
Case closed, one might think. Francis has explained why OAS animates his thoughts by way of proposing a future broad-spectrum flu antigen vaccine.
Except, that one must ask why this was not taken as a bridge to be crossed when arrived upon: Why not create a “universal” vaccine and see if it works first, before arguing about why it has failed?
And why, in the meantime, ignore or memery-hole all evidence that there is no reason to anticipate that such a universal vaccine would be foiled (of all things) by “imprinting”? Why ignore Maurice Hilleman’s glorious success with the Asian flu vaccine, a mere two years prior - doesn’t that imply that any future universal vaccine would be just as effective as Francis’s 1943 PR8 vaccine (which is to say, not perfect, but impressive)?
While a modern reader might be conditioned to understand flu vaccination as a failed cause, our review of the literature demonstrates that the evidence in 1960 was far more mixed, with the most recent experience being one of encouraging success. Moreover, the only evidence supporting the notion that “imprinting” might be driving the difficulties with cross-strain vaccination so far was, as we have just reviewed, Francis’s own ridiculously flimsy work in 1953 and 1956.
And so it would seem that the animus behind Francis’s obsession with OAS was not that flu vaccines were yet self-evidently a “failed cause,” but rather a cause that he, personally, had failed at.
Just as, on his deathbed, a briefly-enjoyed sled became the obsessive symbol of Charles Foster Kane’s mourning of innocence, so a sunset-bound former Rockefeller Institute wunderkind, the first American to recover a flu virus, devised a white whale to explain away his own fall from grace.
Francis died in 1969, his passing apparently accelerated by over-work.27
Original Antigenic Sin is not real. It never has been real. It is the fever dream of a scientific wash-up.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Yewdell, JW. Santos, J. “Original Antigenic S in: How Original? How Sinful?” Cold Spring Harb Perspect Med. 2021 May 3;11(5):a038786.
Davenport, FM. Hennessy, AV. Francis, T Jr. “Epidemiologic And Immunologic Significance Of Age Distribution Of Antibody To Antigenic Variants Of Influenza Virus.” J Exp Med. 1953 Nov 30; 98(6): 641–656.
Reviewed in footnote 3.
In “B Cells Anonymous.”
Jensen, KE. Davenport, FM. Hennessy, AV. Francis, T Jr. (1956.) “Characterization Of Influenza Antibodies By Serum Absorption.” J Exp Med. 1956 Aug 1; 104(2): 199–209.
Unfortunately, the skew to the younger end in the 17-26 group (median age = 18) might have resulted in a high number of individuals who missed the final round of “A” classic in 1943 (despite already being four), due to an interruption with the B virus in 1940. Luckily Francis and Co. provide the raw data on individual donor responses (sarcasm). We’ll have to settle for an assumption that it is “likely” that any 17 or 18 year-olds were primed by A, not prime. Details for subjects by age-group are provided in a separate reference, here.
Note that in the discussion section, long consideration is given to the role that discrete germinal center responses might have in driving the clearly heterologous antibody response that results from repeat influenza exposure (totally echoing my mechanistic argument against OAS in “B Cells Anonymous”). And yet the concluding sentence throws all that out the window.
Whatever the mechanisms may be, it it clear [via outright assumption!] that each antibody response to the various strains is dependent on preexisting immunologic factors and that antigenic similarities are emphasized in human hosts rather than slight antigenic differences which can be demonstrated among influenza viruses. [False, “Team” viruses did not remove new non-Team antibodies.]
Stewart, WH. (1958.) “Administrative history of the Asian influenza program.” Public Health Rep (1896). 1958 Feb; 73(2): 101–113.
Medical Research Council Committee on Clinical Trials of Influenza. (1958.) “Influenza vaccine trial.” Br Med J. 1958 Feb 22; 1(5068): 447–448.
Culver, JO. et al. (1960.) “Antibody Response to Influenza Vaccines Containing the Asian Strain.” J Immunol. 1960 Aug;85:197-202.
Medical Research Council Committee on Clinical Trials of Influenza. (1958.) “Trials of an Asian Influenza Vaccine: Fourth Progress Report.” Br Med J. 1958 Feb 22;1(5068):415-8.
Reviewed in Part 1. The 1945 observational results showing closer to 75% protection are arguably the result of Army and Navy groups being housed separately (so that “herd” protection applies), or of multi-year injections improving response in the current year.
In “B Cells Anonymous.”
Medical Research Council Committee on Clinical Trials of Influenza. (1957.) “Clinical Trials Of Influenza Vaccine: Third Progress Report.” Br Med J. 1957 Jul 6;2(5035):1-7.
Davenport, FM. Hennessy, AV. Drescher, J. Mulder, J. Francis, T Jr. “Further Observations On The Relevance Of Serologic Recapitulations Of Human Infection With Influenza Viruses.” J Exp Med. 1964 Nov 30; 120(6): 1087–1097.
Note that a comment in the discussion section of this paper (which is not about OAS) appears to show the influence of criticism of the school’s preceding work on OAS’s heavy reliance on pooled samples:
The practice of serologic archeology is a hazardous venture similar to the challenge of being obligated to attempt the reconstruction of the skeleton of a dinosaur from a few teeth and some fragments of bone (22). Standing by themselves without reference to the sum total of accumulated information, the reconstructions presented seem at first grotesque. Moreover, unless constantly reexamined as new information becomes available, the models may persist either as unacceptable factitious reconstructions or justifiable credence concern- ing the validity of the mode] may be withheld.
However, as reference 22 is given as “Mulder, J., Virology of pandemic influenza. Facts and speculations, Boerhaave Conference on Respiratory Virus Diseases, Leiden, Holland, April 1962, in press.” it would not appear to be outsider criticism after all.
Overall, it seems if there was anything like vigorous critique against the flawed evidence put forth by the Ann Arbor school for OAS in this early era, it was limited to private correspondence.
De St. Groth, SF. Webster, RG. (1966.) “Disquisitions On Original Antigenic Sin I. Evidence In Man.” J Exp Med. 1966 Sep 1; 124(3): 331–345.
Virelizier, JL. Allison, AC. Schild, GC. (1974.) “Antibody Responses To Antigenic Determinants Of Influenza Virus Hemagglutinin II. Original Antigenic Sin: A Bone Marrow-Derived Lymphocyte Memory Phenomenon Modulated by Thymus-Derived Lymphocytes.” J Exp Med. 1974 Nov 30; 140(6): 1571–1578.
Kim JH. Skountzou I. Compans R. Jacob J. (2009.) “Original antigenic sin responses to influenza viruses.” J Immunol. 183: 3294–3301.
As in “Darmok and the Spike Protein at Tanagra” and “Even-Steven,” reviews of two studies where OAS / “imprinting” are taken as affirmed without designing or adhering to a set of expectations for what results OAS / “imprinting” should imply.
Oshinsky, David. Polio: An American Story. (Oxford University Press, 2005.) Ch. 6.
Francis was president of the Society of American Bacteriologists (later American Society for Microbiology) for 1947. It would therefor seem that the “colleague’s” comment is in regards to:
“Mechanisms Of Infection And Immunity In Virus Diseases Of Man.” Bacteriol Rev. 1947 Sep;11(3):147-56.
This paper seems fine.
Francis, T Jr. (1960.) “On the Doctrine of Original Antigenic Sin.” Proceedings of the American Philosophical Society. Vol. 104, No. 6 (Dec. 15, 1960), pp. 572-578.
Andrewes, CH. (1956.) “Influenza: Theme And Variations.” Calif Med. 1956 Jun; 84(6): 375–380.
(Francis, T Jr. 1960.)
Nathanson, N. (2005.) “David Bodian’s Contribution to the Development of Poliovirus Vaccine.” Am J Epidemiol. 2005 Feb 1;161(3):207-12.

















But what does it mean? I've noticed so much disagreement among those experts in virology. You'd think the immune system would have been figured out by now. The hubris of scientists who would tell us the introduction of strange things like trillions of spike proteins running loose in the body will save us because they know best. Unfortunately, what they know best seems to change over the years.
The "Ann Arbor school." Ha ha ha
Here in Ohio, we also try to avoid uttering the vulgar name of that school as much as possible. Instead we refer to it as "that school up North" (TSUN).