OAS Lit Review / Timeline Pt. 1
History of the "research" into a modern pseudo-scientific myth. Part 1: Meeting the flu.
An appraisal of research into the nonsensical myth of “Original Antigenic Sin” must start at the beginning: The obscure medical quest to harness and understand the flu virus, before it was understood what “viruses” really are. This way, the theory can first be understood in the context of the knowledge and methods of its own era. Readers not interested in a philosophically-tinged, steampunk sojourn through scientific history should skip this part.
Part 2 will pick up in the modern era, when the myth was mysteriously revived from obscurity.
In case of later editing or expansion, a changelog for this post will be maintained in the footnotes.1
Introduction: The Author Has a Take
Reviewing the history of research into OAS should be easy, even for someone with such schizophrenic research habits as myself, since the brunt of the legwork would seemingly have already been completed by Yewdell and Santos in their 2020 review:2
However, that essay doesn’t quite satisfy when it comes to the early history of the research. And further (as Yewdell and Santos grapple with), any discussion of modern flu research is plagued by the problem of inconsistent application of the very term in question.3 As the title - “How Sinful?” - implies, modern discussion of the myth often disowns rigid adherence to the obvious implication that OAS is bad.
Part 1 of this essay is my attempt to be far more precise, thorough, and outsider-friendly regarding the development of flu research methodology and understanding up to 1953, as this context is critical for appraising the original observations that inspired the myth.
Age of Enlighten-meh
A history of flu research up to 1953.
1802 - 1899: A Rofe By Any Name
In the era before microscopes and germ theory, “virus” was simply a placeholder concept for an unseen agent that transferred from one being to another, conferring the first’s disease to the second. With pox, for example, it meant simply, “whatever was in the pus” that spread the condition from one beast or man to the next.
Upon the advent of microscopes, “virus” was still a necessary placeholder, describing whatever still could not be seen by a microscope and could not be cultured in nutrient mediums, and yet just as before could appear to be transmitted between beast or man. In the mad rush to ascribe to every apparently contagious disease a particular bacteria, it remained the case that some diseases, including smallpox, were not associated with any. And so “virus” remained the placeholder concept for whatever entity, non-bacterial, was transmitting these diseases.4 However, such determinations were intrinsically perilous, given that culturing bacteria was (and remains) an imprecise art.
Determination that a “virus,” not a bacteria, was responsible for a disease could be more precisely litigated by filtering fluids through porcelain filters (typically eliminating bacteria). If fluids from a diseased animal or human could still pass symptoms on after being porcelain-filtered, then “virus.” This was achieved at first for smallpox, foot and mouth disease, and rabies, and in an imperfect form for polio by Karl Landsteiner in 1908.5 But in-practice adherence to filter-passage was not stringent for flu at all, as we will see below.
In 1889, at all events, when the illness humans describe as “flu” made its reappearance after many decades of absence, it was not attributed to a virus, but to a bacteria. This was the way biologists preferred their microbes, at the time.
Timid New World
As we head into the 20th Century, it is important to understand it in the context of the prior one; man did not gain a new way of seeing the world overnight. The researchers of the early 20th did not grow up with “computer-generated,” colorized depictions of the things they were studying, to poison their minds against other possibilities. Nor had microscopes proved able to illuminate the world of disease completely; for the rest, the naked eye still served just as well. And so “virus” remained, in the dark ages of the early 20th, a provisional label for an unseen agent - sometimes a “particle” - that caused effects in experiments that were observed on the macro level. In 1928, Griffiths, in regard the material responsible for transferring mouse-killing-ness from dead bacteria to live bacteria like a mouse combat tactics manual (i.e., DNA), would describe a “transforming principle.”6 For “virus” it was no different. True, attempts were sporadically made to establish whether viruses could be treated like bacteria in the real-world contexts of transmission or sterilization,7 or to measure the size of given viruses via filtration, clearly betraying an inflexible germ theory mindset - but in lab passage a virus was treated no differently than one would treat a ghost believed to reside in a grandfather clock, or spirit energy gathered in ley lines.
It is therefor absurd for a modern critic to attack the work that follows by claiming that germ theory, or virology, or a cabal at the Rockefeller Institute, imposed a predetermined outlook on phenomena easily explainable by other means. Firstly, the work was performed in advance of offering an explanation for what was occurring.8 Secondly, virus recovery was understood to be one of several imperfect arguments in favor of a relationship between the lab product and the human illness, especially given that illness only tended to be associated with virus recovery on “epidemic” flu years when many people were ill; on “off” years most of what was reported to be flu resulted in no recovery.9 Moreover, the methodology of early research would not evince a presumption that some static, singular thing was being “isolated;” even if the i-word was often liberally employed. Instead, recovered viruses were understood to require perpetual repetition of the proof of their potency and the ability to be disarmed by blood serum from previously exposed animals. A classic example is Shope’s first demonstration of passage of his swine flu strain into ferrets, in which each passage is understood to be potentially relevant to what is being studied:10

Likewise, lack of cross-protection in serum collected after encounter with different strains was used to validate the specificity of immune response, but also affirmed the self-referentiality, or programming for self, of “viruses.” And finally, alterations in performance of “viruses” after being introduced to passage mediums was taken to demonstrate that “viruses” adapt. None of this required defining or understanding what a virus was; and the habit of treating strains as static, singular objects would only emerge later.
To insist the researchers of the early century dismiss their own observations (on the grounds that the agent they observed required perpetual renewal) requires assuming that Nature operates by some sort of axiom that life is not transient and interconnected - that nothing in biology can be believed to exist unless it is within the powers of man to fix it in place with the precision of a pile of magnesium salt. This is an assumption with no foundation; a superstition at best.
1928-1936: Easy Mode
In 1889-90, flu made a widespread reappearance after what is believed to be a 50 year period of not contributing to illness or mortality.11 During this outbreak, a bacteria, Haemophilus influenzae, was associated with the disease by a German bacteriologist.12 During the 1918 Spanish Flu, especially the first wave in the spring, the same bacteria failed to attend the disease consistently, throwing the etiology of the thing humans called flu into question.13 Was the flu a “virus,” after all?
Swine, 1928-31
In the American Midwest in the late summer of 1918, an illness overtook pigs that generated similar symptoms to human flu, a novelty which astonished local veterinarians.14 This returned in subsequent autumns, with swine mortality varying by the year. In 1928, 29, and 30, Rockefeller Institute researcher Richard Shope succeeded in prompting illness in pigs back in New Jersey with mucus, lymph, and lung extracts from flu-struck pigs in Iowa, and in serial-passaging the same illness to further pigs by shoving snot or lung material from prior pigs into other pigs’ noses, or by live contact, resulting in eight successfully-maintained strains.
Although skipping any filtration step, he confidently described the agent as a “virus” when publishing his results in 1931.15
The representative strain of this series, “strain 15,” later annotated “SW,” would turn out to be sensitive to sera from almost all humans alive during the Spanish Flu, having apparently not changed its physical chemistry (“antigen”) in the intervening years. However, children born after the early 1920s would show no such immune memory, except in the first months when maternally donated antibodies are present.
The Snot Heard Round the World: WS, 1933
Influenced by this work and his group’s own ongoing research into Canine Distemper Virus, Wilson Smith at the UK’s Medical Research Council at Mill Hill attempted to replicate flu symptoms by dropping some of then-ill Andrewes’ (filtered) throat washings into the nostrils of some ferrets. Both the target animal and the route of administration were novelties invented by Smith, per an un-cited biography of the man.16 This near-failure (the ferrets died of distemper instead, and the strain was lost) was reversed when Smith himself came down with flu (possibly from the ferrets), and reattempted the experiment.
This time the ferrets took ill with flu-like symptoms, and either ferret-to-ferret contact or the (filtered or unfiltered) ground-up nasal turbinates from ferret n could reliably produce the illness in ferret n+1, establishing passage. Serum from recovered ferrets and humans, if mixed with the nasal virus smoothie, was further shown to prevent this type of transmission.17 Thus was born the “WS” flu strain,18 which lives on to this day as a primarily mice-adapted virus and a volunteer on race days.
Further, the Mill Hill team’s endeavors to demonstrate that passaged material was both infectious and bacteria-free after filtration ushered in consensus that the flu was caused by a “virus.”
PR8, Phila, Melbourne, 1934/5
Back in New Jersey, the Rockefeller Institute’s two Thomases were hot on the Brits’ heels, with Francis and Magill independently recovering two human flu viruses the next year, from a patient in Puerto Rico and Philadelphia.19
Again, one of these - Francis’s “PR8” - remains in use in research as a mouse-adapted strain.
The next generation of strains, starting from 1937 onward, would less frequently feature a ferret to mouse transfer. For this reason, WS and PR8 came to be considered less reliable time-capsules of the 33/34 flu’s physical chemistry (its “antigen”) vs. later strains.20 On the other hand, PR8 retained high cross-reactivity to serum based on 1935-46 A-strains whereas WS did not, leading to rather different treatment: WS was speculated to represent the twilight of a previous era in flu’s physical chemistry (“antigen”), from between mid-1920s and 1933.21 PR8 was taken as the most immunogenic and lab-reliable representative of the “A” strain of flu as later defined, and was regularly used for early flu vaccine trials as late as 1951.22
A year later, Burnet, a future heavyweight in immunology, put Australia on the map by producing a long-serving Melbourne 1935 strain.23
Passage Me With Care: Ferrets, Mice, Eggs, 1935
These seminal human flu strains could at first be maintained by repeated, timely passage through ferrets of (preferably filtered) nasal or lung material from previously infected ferrets, as was being done with swine flu in pigs by Shope, and likewise with mice. Shope, building off of the work on human flu, demonstrated that his representative swine strain could be passaged to ferrets and then mice as well.24
At first, ferret-passage was found necessary to improve the odds of successful passage to mice. By 1937, it was clear that human flu could be transferred directly to mice, but that it took a few “blind-faith” passages until the virus adapted to the new host (hey there, gain of function) and caused visible lesions.25
By 1935, both Smith and Francis/Magill had made baby-steps toward tissue culturing of their flu strains, independently finding success with minced chicken embryo and a nutrient solution (technically producing an embryo-tissue adapted version of the virus, but which still generated illness in mice).26 Burnet’s Melbourne strain was likewise first recovered in ferrets in 1935, passaged in mice until 1936, transferred to egg, and finally deep-frozen (as egg juice) in 1940.27
DIY Mouse-Protection Assay
28By making an interruption in the mouse passage chain, our pioneers could evaluate immune response to glean several early insights. You can try at home: Before taking your infected, ground up, filtered mouse lung fluid and rubbing it in another mouse’s nose, mix it in with blood serum from a previously infected ferret, rabbit, horse, or human. Then see if the mouse still dies (a good idea is to use several mice and confirm something like consistency in the results). For an added touch, dilute protective serum step-wise and see how diluted it can get before it stops protecting the mice - this dilution is known as a “titer,” and can be used to express and compare the potency of protection.
The donor serum, when mixed with the to-be-passaged lung smoothie, will keep the next mouse alive if it has little invisible things in it (“antibodies”) that stop the virus from working. If not, the mouse will die.
A human donor’s serum mixed with PR8 will protect the mouse if it has PR8 antibodies. Or mixed with SW, will protect the mouse if it has antibodies against Shope’s swine flu. Or both, if it has both.
A PR8-inoculated ferret’s serum will protect the mouse against PR8, presumably, as well as WS and SW if they are also targeted by the antibodies against PR8. And vice-versa. Asymmetry is also possible: Maybe ferrets make cross-protective antibodies in reaction to one strain, whereas the cross-protected strain doesn’t protect against more than itself. And inconsistency is par for the course: Early on, it was not clear whether PR8 and Phila are alike, as using ferrets and rabbits to generate serum to mix in with lung juice would generate inconsistent patterns of dead mouse. And ferrets with strong or weak immunological responses could create false patterns of relationships between strains.29 The only cure for these limitations was patience and hard work.
Mice excel for this type of testing for numerous reasons, among which: They are easy to acquire and handle. The virus, once mouse-adapted, may be devastating for the lungs, but doesn’t rebound into the nasal cavity as with ferrets. This eliminates natural mouse-to-mouse transmission, whereas with ferrets it is necessary to take pains to isolate them from each other, and isolation is probably not always successful (plenty of surprising and inconsistent results were derived from ferret sera in cross-immunity tests). And, circulating human flu doesn’t pre-generate an immune response in mice via accidental exposure, as once again complicates things with those stupid ferrets.
Early Insights in the “One Flu” Era, 1936
Humans alive in the early 1920s were still immune to the 1918 flu, which was different than the current one.
Shope argued from the start that swine flu was a descendant of the 1918 flu. In 1936 he produced startling results showing that human donor sera for most age groups universally or commonly protected mice against his swine flu - except in children born a certain time after 1918 (for newborns, maternally-donated antibodies could protect the mice):30

Shope further demonstrated that many donors were protective against his swine flu, but not the human flu captured by Francis - and so swine flu protection was not a result of recent human flu immunity. Smith and Co. at Mill Hill were not so easily convinced, but these results would be replicated numerous times in the future, including by Francis in 1953 in arguing for OAS.
(The dominate theory for why swine flu was still such a precise match for apparent 1918 flu antibodies would be that the annual slaughter of pigs ensured there was no immune pressure for the virus to escape from.31 This, in turn, is one of the stronger pieces of actual evidence that antigenic variation is necessary for natural flu’s survival, rather than just a creative quirk.)
Current human strains were closely related to each other, and distantly to swine flu
Here we employ the mouse protection assay as previously described. If I make a flu strain, and you make a flu strain, and sera from ferrets recovered from my strain can robustly protect mice exposed to your strain, and vice-versa, our strains would appear to be antigenically identical. On the other hand, if your strain showed cross-protection with a third strain, but mine different, it might indicate that yours is a more wide-spectrum representative of the family in which all our strains reside. And a group of some other people might have a different family that doesn’t cross-protect mice with ours very well at all.
For human and swine flus, there appeared to be some overlap - ferrets were protective in one direction, but not another.
For WS, PR8, and Phila, on the other hand, cross-protection at first seemed robust when compared with horse serum shipped by Smith and co., even though the first strain was recovered in the UK and the other in the Americas. In light of how quickly the handful of other passaged viruses to date had revealed multiple serotypes, hopes were high that the flu virus would prove to have only one type. However, this cross-protection did not hold up on repeat testing with ferrets.
Human sera is protective after a recent flu recovery, but not during the acute phase.
In the 30s, there was still something not-quite-certain about the theory that antibodies were in all cases an immune response.32 Deciphering this question might have proved particularly difficult for flu, given that diagnoses on off-years tend not to involve any recoverable virus. The critical demonstration of the relationship between infection, recovery, and mouse-protective antibody was supplied by Francis and Magill.
In their counterpart to Shope’s swine flu immune survey, they demonstrated that anti-PR8 immunity among the same donor pool was widespread except in the youngest children (age 1, who would not have had natural encounter with the virus thanks to maternal antibodies during the last flu season), but not universal:33
But the more impressive finding had already been published the year before, involving three influenza patients being treated at the Rockefeller Institute hospital. Sera from these patients protected mice after recovery, and up to 6 months afterward, but not during the infection itself. This was evidence for seroconversion, the classical marker of memory immunity:34

The set-up for vax success?
And so, the problem seemed simple. Get all currently un-immune humans to make antibodies against PR8, and flu is a goner.
Of course, questions remained, including why anti-PR8 protection was found in so many donors that had no memory of recent flu (about half), and why flu season was still a thing at all if antibodies against the latest strains were already so widespread.
And one could certainly argue that the age-delineated recognition of Shope’s swine flu was clear proof that flu was an evolving beast; that it had changed, and therefor would change again. And, to be fair, there were inklings.35 But the British clique had not yet been sold on Shope’s conviction that he had recovered an antigenically pristine copy of the 1918 flu. Perhaps, they speculated, humans simply developed cross-protection against the swine version of the virus once re-exposed to human flu enough times.36
They were wrong.
1937 - 1947, Part 1: Future / Perfect
Before continuing with the history of the virus before “OAS,” a discussion of how methods would evolve during the same era.
As the above history should suffice to supply the missing dots which are left out of the normal cartoon narrative about flu research - since immune escape, antigenic drift, and flu vaccine failure are common knowledge - the reader may only want for a discussion of those research methods which were added to the standard toolkit after 1936, before advancing to Part 2 to evaluate the research surrounding the myth of OAS.
Back to the Egg, 1937-40s
Early developments in culturing flu in eggs deserve mention.
By the 40s, four different methods of culturing the virus in fertilized hen eggs were in use, and successful recovery of flu from human samples could be reliably produced by blind-faith passaging with one method and then switching to an easier one after the virus adapted to egg. Even better, adapted material was essentially ready-to-use as an injectable product which one could label a “vaccine,” even if completely unlikely to prevent observed experiences of flu. As Andrewes (of the first human-to-ferret throat washing transplant) summarizes in 1949:
Four main methods of infecting fertile eggs are in use: onto the chorio-allantoic membrane, into the amniotic cavity, the allantoic cavity, or the yolk sac. In studies of viruses each method has its own uses (Beveridge and Burnet 1946). For primary isolation of influenza virus from human material, amniotic inoculation is much the most sensitive method. Garglings need not be filtered: one does better by adding penicillin (with or without other agents) to suppress bacterial growth. But if you should ever try to isolate virus thus or send it to a laboratory, do please keep the material cold and see that it reaches the laboratory within an hour or two. Garglings sent through the post are often most unappetising by the time they reach their destination. The amniotic technique takes a little practice, but after one or two passes by this method, one can usually induce the virus to grow in the allantoic cavity. Allantoic inoculation is singularly easy, requiring little more than selection of a suitable spot on the egg surface through which to introduce a needle. Well-adapted viruses reach high titres in the allantoic fluid and this material is used as the source of virus for making formolised [formaldehyde-treated] vaccines.37
For context, other human viruses found amenable to egg passage between 1930 and the late 40s were vaccinia (i.e., animal-pus-derived smallpox “vaccine” virus),38 variola (human-pus-derived smallpox virus), mumps, and herpes simplex; while the first “common cold” virus was not recoverable until true cell culture methods improved at the turn of the 50s.39
Naturally, eggs also began to be used for the flu protection test, i.e. neutralization assay, in place of mice.
Assay HI, 1942
As flu strains multiplied, so too did the work of putting throat juice into eggs, validating cultured strain pathogenicity in animals, and taxonomically mapping strains by their physical chemistries (“antigens”). It’s doubtful the mouse protection assay would have come close to keeping up. Fortunately, a discovery by George Hirst forever redefined the way humans obsess over fluid collected from eggs they have previously put throat juice into.40
As elegantly summarized by Andrewes:
An important step forward was made when Hirst (1942) discovered that influenza virus in high concentration would agglutinate the red cells of fowls and other species. This finding is the basis of a quantitative test [also developed by Hirst] with which to estimate the amount of virus in a fluid.41
“Agglutinate” means to make red blood cells glom together. “H” (for “hemagglutination,” or blood-glomming) was chosen for the term for pouring step-wise dilutions of stuff into wells combined with red blood cells until they either caused (“HA assay”) or failed to inhibit (“HI assay”) cell-glomming. Thanks to this assay, “H” was also attached to the “antigen,” or rather fusion glycoprotein, on the flu virus that was later determined to bind to the relevant receptors on red blood cells (the “H” in the “HxNx” flu classification scheme).
“Quantitative,” meanwhile, is more than a poetic flourish: It reflects that the HA/I assay (like the mouse neutralization assay) could go beyond merely affirming that virus or antibodies are present, but give an idea of how much.
Hirst, in his early work validating the assay, could step-wise dilute the serum being pre-exposed to a dose of egg-passaged flu, for example, and observe at which dilution (“titer”) of serum the serum-flu combination made red blood cells glom together (meaning, the serum failed to inhibit glomming). He further demonstrated that this dilution corresponded with only a bit of skew to the serum dilution at which a serum-flu combination would make mice dead (meaning, the serum failed to prevent death). This strongly argued in favor of the conclusion that the more you could dilute serum before mixing it with flu made glomming, the more antibodies specific for that flu strain were in the serum.
Later iterations of the HA/I assay would include frequent preparation of samples with “receptor-destroying enzyme (RDE)” - a term first in reference to what was later discovered to be neuraminidase on the flu virion, and later and more frequently a separate chemical cultured from Vibrio cholerae.42 “Destroying” the target substrate of haemagglutinin with a bit of RDE is useful for reducing sensitivity of a given assay or constructing more elaborate inhibition designs.
Back to Andrewes:
Further, the [glomming of red blood cells exposed to flu] is inhibited by appropriate antibodies; an A virus by an anti-A and B by anti-B, so that one has also a specific quantitative test for antibodies. Hirst's haemagglutination test has two practical applications [in the context of recovering new circulating flu strains]. [1] With it one can determine whether one has successfully isolated a virus in one's egg and [2] if so which virus. The amniotic or allantoic fluid of the egg inoculated with human material is put up in dilutions in tubes against suspensions of washed red cells and agglutination looked for after an hour's standing on the bench. If it is positive and the presence of virus can be deduced, samples of the agglutinating fluid are mixed with anti-A, anti-B and control sera respectively, and a quick diagnosis is possible between A and B virus according to which serum inhibits the haemagglutination.
And so Andrewes makes no comment on typing strains within the A or B genera with this new assay. He further promotes the more difficult complement fixation test to newer researches, as being less prone to beginner mistakes. Andrewes, in this marvelously plain-spoken overview, is writing to an audience of presumed neophytes. The author’s choice here is a wise one, as his field is no longer an elite pastime shared by a handful of colleagues, and more and more a shark-bait free-for-all.
The HI assay only accelerated this change, democratizing flu research in the same way pianos extended musical performance to the middle class home.
House of Cards, 1947 - Present
And so while it might seem that a structured plate of wells would have to be more precise than a room full of sick mice, the hazard was that flu researchers would no longer have to acknowledge the contingent, ephemeral nature of their own results. To be sure, HI assay results would routinely be duplicated with neutralization tests (egg or mouse), complement-fixation tests,43 or both. But the hazard for positive reporting bias should be obvious: HI assay results contradicted by neutralization tests would, potentially, simply not be reported, rendering this method of verification essentially a rubber stamp. Whereas the passage era forced researchers to speculate on how their results might be distorted by ferret and rabbit immune differences from man, the HI assay in practice is taken as an objective reporter of the human immune system.
Today, as an age of interest into the genetic mechanics of flu resides, vaccine research resurrects the obsession over that which man is most easily able to measure - the antibody - after a further 8 decades of discoveries into the complexities of the immune system, and of the Antibody Obsession’s failure to produce reliable predictions of immunity. And so the HI assay, by making flu virus and antibody measurements more precise, had robbed scientific investigation of the flu of both humility and creativity.
The “passage era” - the era when the ambiguity and instability of passage was by necessity acknowledged - was elapsing in other ways. The drive for a human vaccine demanded that the “infectious material” being passaged behave like a quantifiable biological product, i.e. a “virus” which could be measured, purified, inactivated, adjuvanted, and cross-strain-combo-packed in the hopes that this would rescue flu vaccines from their current troubles, whether those were intrinsic or a consequence of the virus’s mutability. Egg culturing lent itself to consistency but not to purity; and so the early 40s saw intensified interest in centrifuging, freeze-thawing, and red blood cell glom-and-release methods of “purifying” the product of egg proteins, with the assistance of new electron microscopes to evaluate the results.44 Regarding the agglutination method, it was found that red blood cells could glom virus at cold temperatures, at which point the rest of the egg material could be removed - and then the result simply brought to room temperature to release glommed virus.45
Perpetual failure to generate a “universal” flu vaccine could only contribute further to the ossification of the field. As the virus outlived the knights who first tried to slay it, their progress in understanding the problem had to be transferred into new minds, en mass. Flu was no longer fought with by a few lone souls, but by an army; and armies require discipline and uniformity of approach. And so it is no wonder the influenza apparatus of an Organization absurdly purporting to represent “World Health” has been directing efforts from on-high since 1947.
In this process there was no room for nuance, and so dogmatic thinking about the problem took the place of earnest reevaluation of the observations to date. Besides being reflected in the dull, imprecise materialism of the language of modern flu vaccine research, this inflexibility of thought is reflected in the eternal catastrophizing of the potential for another 1918 (thereby brushing aside all the puzzles about that event’s mortality and epidemiology that were still obvious to researchers in the 30s46).
Meanwhile, many of the central biological mysteries of flu which captivated the minds of two of those aging knights, Shope and Andrewes47 - how does flu spread more quickly than direct transmission can explain, and yet the entire world often produces identical strains on a given year? how is it that early summer outbreaks often preview the strain to emerge during flu season proper? - have languished, rarely being acknowledged in science today (there are of course exceptions48).
Instead it is the aging Francis’s obsession - OAS - which has undergone a modern renaissance: And of course it has, not because it does anything to explain observed human outcomes with flu itself, but because it deals with antibodies.
1937-1947, Part 2: The Hydra
Antigenic drift rears its head, followed by a second genera of recoverable flu, and finally, the first recorded antigenic shift.
Three is a Crowd
On the other hand, we have observed that some strains of virus isolated in England in 1937 are not identical with the W.S. strain with which we have worked since 1933 and which we used to prepare our vaccines [for a failed 1937 trial]. The difference is revealed by titrations of ferret sera as well as of rabbit sera, and also in cross-immunity experiments. There is a considerable antigenic overlap, and the strains are more closely related to each other than to the swine-influenza virus; but there are distinct differences. The importance of differences between strains to prophylaxis and to epidemiological studies one can at present only guess at. The tangle is not going to be an easy one to unravel.49
-CH Andrewes, 1937
And so, it all began to fall apart.
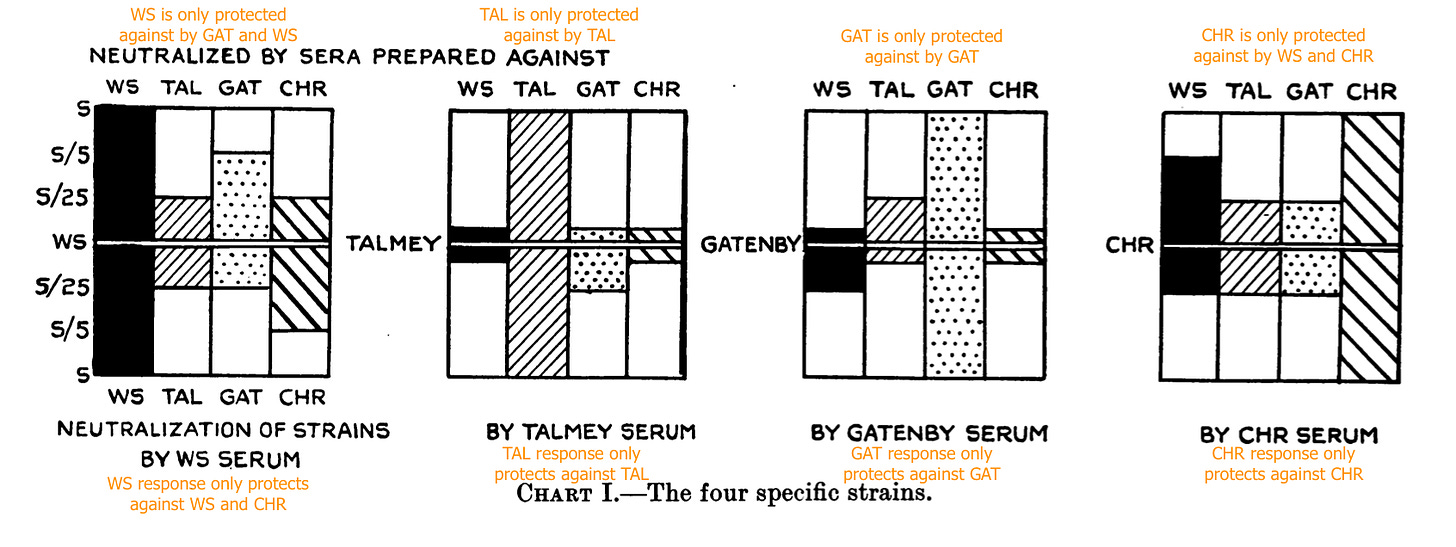
1937 was a bumper crop for recovered flu viruses, either with ferret-to-egg or direct-to-egg methods, especially in Britain. Comparing protection of recovered ferret sera between these newly recovered strains, and further refining their results by titering (step-wise diluting) the sera to see when protection ends, Smith and Andrewes found most 1937 flus to be only distantly cross-protective with their snotty lovechild WS.
What’s more, they weren’t perfect matches for each other, once these more precise measurements of mouse protection were used. Altogether they identified four major groupings in that single crop, doing their best to double-check these relationships against the considerable noise arising from extra-strong or extra-weak ferret donor immune responses:50
The rest of the strains they tested tended to either be a WS match (a result possibly resulting from lab contamination with lingering WS51), a bridge strain apparently halfway between two of the four, to be generic, or to give no clear result. The authors regarded as more worth discussion the appearance of the 3 new clearly non-overlapping strains.
Flu’s physical chemistry (“antigen”) was evolving, as if to evade the immune response of the previous years as represented by WS.
Plan B
In 1940, a virus not showing any cross-protection with any of the previous strains was recovered during a flu wave. This prompted the designation of the previously-familiar group as influenza “A” viruses, and the new discovery as “B;” a move which proved to hold up to modern taxonomies based on the genetic structure of both classes. B was found at the time not to drive mortality very much (it was also retroactively credited for the 1936 season), and continues to behave this way today.
Meanwhile, a representative recovered strain from the 1940 bug, “LEE,” became a regular in experimental vaccines in the years to come. (Typically, these trials would give recipients both an A and a B injection, in the case that either of them were kind enough to show up that winter).
The era of B’s discovery, between 1937 and 1947, nonetheless remained peaceful, as summed-up by Andrewes:
In recent years it has been possible to isolate viruses from each epidemic and thus to discover much more than is possible by the mere study of vital statistics. Since 1940 we have known that there are two influenza viruses, A and B, having nothing in common antigenically, and therefore likely to behave epidemiologically as separate entities. […]
What seems very clear is that major influenza outbreaks can mostly be ascribed to influenza virus A. Great interest therefore attaches to the antigenic variations which have been occurring among the A viruses since 1933. […]
During the decade 1936-46 the influenza strains isolated from all parts of the world were more or less closely related serologically to the PR8 strain recovered from Puerto Rico by Francis in 1934, and to the Melbourne virus isolated by Burnet in Australia in 1935. Within this group there were minor variations such as those detected in southern England in 1936-7 (Stuart-Harris et al. 30). Also, in 1943, the Weiss strain was sufficiently different from PR8 for homologous (Weiss) vaccines to give particularly good results in a vaccination trial in the USA (Commission on Influenza 3). Still, all the strains from this decade were fairly closely related.52
What’s of note here, is that influenza A didn’t make much of an appearance for the next two years. It seems as if the evolutions of the virus in 1937 and 1943 were not themselves enough to keep the virus in heavy observed circulation.
As this “quiet” was preceded not by a storm but a drizzle, the first endeavors in flu vaccination churned ahead with almost as much confidence as might have characterized the outlook in 1936. But at the forefront was a researcher not prone to confident pronouncements of any kind,53 Thomas Francis Jr.
Home Run, 1943
In the early 40s, Thomas Francis was no longer a pioneer in a new realm but the don of American flu vaccine research. Leaving Rockefeller in 1941, he helmed the newly-minted Virus Laboratory at the University of Michigan. The year later, his lab was tapped by the Army to produce a vaccine for American soldiers against ignoring radar blips flu, and issued some of the National Foundation’s infinite polio budget as a grant for generically “training virologists and studying virus diseases,” all of which went on top of a grant from the Rockefeller Institute.54 And he had, under his wing, a hand-picked, ambitious protege who shared his preferences for “inactivating” and then adjuvanting vaccine viruses, and as determined as anyone on Earth to test new methods for doing so: Jonas Salk.
Francis was thus not only flush with funds and young talent to parasitize, he had first dibs on the Army’s pool of free, forced test subjects. From the gate, his trial would be a quantum leap in terms of scale and the resulting statistical power he would be able to affix to his results. If anything was going to right the ship in humanity’s attempt to slay the flu virus, it would be this.
Regarding that first generation of attempted flu vaccines, Francis would later write (bold headings added):
1936
The initial studies on vaccination of human subjects with influenza virus were undertaken to determine whether the administration to man of active virus by the subcutaneous or intracutaneous routes would incite infection, and in order to gain evidence of the degree of antibody response which could be elicited. Utilizing the PR8 strain of type A virus it was found that virus propagated in chick embryo-Tyrode's culture, given by either route, did result in the production of neutralizing antibodies to titres comparable with those observed in convalescent patients (Francis & Magill10,1). […]
Stokes, Chenoweth, Waltz, Gladen & Shaw57 prepared Berkefeld filtrates of [Shope’s] swine virus and of human virus A [PR8] from 10% mouse-lung suspensions, and then inoculated members of a children's institution with three doses intramuscularly, while retaining as controls an uninoculated group approximately twice as large. During an outbreak of respiratory disease in February 1936, they recorded an incidence of 12.5% and 12.4% of febrile illness in the controls and in the groups vaccinated with swine virus, respectively, but only 2.7% in those receiving the PR8 vaccine[…]
1937
In the same winter Smith, Andrewes & Stuart-Harris 56 attempted a controlled prophylactic study, among military forces in England, of subcutaneous vaccination with filtrates of a 10% suspension of the WS strain from mouse lung, inactivated by 1:2,000 formalin. Thedisease occurred before vaccination was fully carried out, and the incidence was low. No effect of the vaccination was noted. A polyvalent vaccine similarly prepared was employed in 1938-9, but no evidence of protection was observed in the group under study (Stuart-Harris and co-workers 59,60). Taylor & Dreguss 61 observed no significant effect in their study of vaccinated and unvaccinated individuals, and attention was drawn to the fact that the epidemic strain differed from the WS strain of the vaccine.
1937-41
The next extensive studies were those of Horsfall, Lennette, Rickard & Hirst 30 with material prepared from chick-embryo tissue previously inoculated with PR8 strain of A virus and a strain of canine distemper virus [?]. The minced embryo suspension was inactivated with 1:4,400 formaldehyde, and 1.0-ml doses were given subcutaneously to individuals in a number of institutions. From 30% to 60% of the populations, totalling some 16,000, were vaccinated; the others served as controls. Although a difference in total incidence between vaccinated and controls was observed during an epidemic period, there was a significant reduction in only two of ten vaccinated groups; in two other groups the incidence was higher among the vaccinated than among the controls. The ineffectiveness was attributed, in part, to the lack of potency of one large batch of the vaccine.
Brown et al., 1 using a similar preparation, reported an inconstant but final reduction in incidence from 25% in the controls to 13% in the vaccinated groups. Dalldorf, Whitney & Ruskin, 2 in a limited study of the same material, noted no difference. Siegel et al. 52 employed different vaccine preparations through three successive outbreaks of influenza A in 1937, 1939, and 1941, but observed no difference between vaccinated and unvaccinated groups.55
As inconsistency, potential lack of potency, and strain mismatch, were common themes in analysis of the failed trials, Francis and Salk paid great attention to concentrating the egg-derived product used in their Army trial, incorporating the latest work by Hirst, though these methods were still in a primitive state. Nonetheless, measured antibody levels appeared to suggest adequate quality control.
In the 42/43 season, when a PR8 (A) + LEE (B) vaccine was trialed among 8,000 soldiers, no flu showed up. Francis and co. pivoted to challenge trials during the off-season, from which they were able to report consistently higher rates of infection among (blinded, buffer-injected) control soldiers, especially vs. B.
Off-season 1943 PR8 + LEE Army challenge trials
Illness or fever after challenge vs. sprayed A strain (PR8-adjacent)
Control: 18/36 (50%)
Vaxxed 4.5 months ago: 9/28 (32.1%)
Vaxxed 2 weeks ago: 6/38 (15.8%)
Illness or fever after challenge vs. sprayed B strain
Control: 11/27 (40.7%)
Vaxxed 1 to 4.5 months ago, or double-vaxxed: 8/79 (10.1%)56
Undaunted (their lab was being too well-paid to be daunted), Francis and Salk went to bat the next year with a similar core methodology, 50% bigger test population, and a double-A (PR8 / Weiss) + B (Lee) preparation. This time, nature sent the ball right down the middle of the plate.
43/44 flu season PR8 + Weiss + LEE Army trial
Hospitalizations for flu
Control: 441/6,211 (7.1%)
Vax: 138/6,263 (2.2%)
Unlike in the early British trials, the new injections appeared to show (eventual) potency even if flu arrived before enough time for seroconversion (emphasis added):
Since in two locations (Hale & McKee; 20 Hirst, Plummer & Friedewald 26) the epidemic began at about the time of vaccination, the curves of incidence of disease in vaccinated persons and controls could be followed. In the first week no differences were observed, but after six or seven days the curves diverged sharply as the incidence in the vaccinated group decreased; this indicated that the prophylactic effect of vaccine began at a time when circulating antibodies are ordinarily beginning to rise.57
In 1945, the injections circled the bases again, vs. an outbreak of flu B. The Army at this time had rolled out universal injection; but personnel in university could be compared to naval students at the same locations:

There was no question about it.
Thomas Francis, first American to recover a passage of human flu a decade prior, had conquered the virus.
Until, that is, the virus conquered him back.
A-Prime, 1947
In the 1946/47 season, Francis’s vaccine was being observed in a University of Michigan trial of 17,943 participants and by general illness rates in the wholly-vaxxed Army; and a more retro Mel / PR8 formulation was simultaneously under a massive trial in the UK.
No protection was observed anywhere. That year flu was just as rampant among the vaccinated as not; as if at the stroke of the clock, the vaccine had turned back into a pumpkin.
It was soon obvious what had likely occurred: A new version of A had arrived. It was soon designated “A-Prime” (as in A’). Like B, it didn’t bring any great amount of destruction in its wake - the 47 wave was considered mild. Unlike with B, a detectable resemblance with A could still be found in the form of cross-protection against PR8 after infection; but otherwise the new strain was more distinct than anything recovered before, including Shope’s swine flu.
Worse, strains resembling PR8 would turn out to vanish from circulation, rendering Francis’s vaccine and all previous recovered human strains permanently obsolete. The flu had performed its first observed antigenic shift.
Returning to Andrewes:
Then in 1947 vaccines used both here [8] and in America quite failed to give any protection. The A viruses isolated were decidedly remote antigenically from those used to make the vaccine, and there is litle doubt that this fact explains the failure of the vaccines. Where were we to go from here?58
And in another review:
In 1946 in Australia, a strain (Cam) was isolated, and proved to be somewhat removed antigenically from those previously prevalent. Its significance was not at first appreciated, but in 1947 similar viruses were recovered in numbers in North America and also from Europe (Sweden, the Netherlands, and Great Britain). In the USA, these strains were called A-prime (A') strains, to indicate a major divergence from the clasical A viruses; strain FM1 from the USA is regarded as a typical representative. Since 1947, strains related to FM1 have been recovered from A outbreaks in every continent, and seem, quite suddenly, to have completely replaced those related to PR8.59
Answering the question of where humanity would go from there, Andrewes describes how A-Prime closed the book on lone gun flu research, and ushered in the dour era of endless, centralized virus surveillance that we live in today:
An unofficial group considering the matter at the 1947 International Microbiological Congress in Copenhagen felt that study of virus strains on an international basis was called for. As a consequence of its recommendations, the World Health Organization asked the Medical Research Council to set up in London a World Influenza Centre to promote co-operation in this field. Its objects were to collect information and, more particularly, strains of virus from epidemics anywhere in the world. It was thus hoped to learn how far apparently new strains of virus caused outbreaks by travelling from country to country, and to attempt classification of influenza viruses on an orderly basis.60
And so the golden age of “one flu” was over for good. Soon, in fact, entire new species of the flu spike protein would appear from the yet undiscovered animal reservoir.
In the “A-Prime” decade, the last years of H1N1’s natural run, it was no longer possible to deny that Shope’s swine flu represented the 1918 virus. Thus it was understood that the virus had changed from swine-like into something closer to WS, then into PR8, and now finally to A-Prime — and these changes defined the breadth of immune memory that could still be reliably discovered in the blood of individuals alive when older strains were prevalent. In like manner, it was understood that the stability of swine flu in pigs is a result of yearly slaughter which erases immunity; thus the instability of flu in humans is a result of immune pressure against the virus.
Flu would never stand still for a human vaccine.
Flu: The Lost Years
Part 1 of the Unglossed OAS Lit Review dealt with the early history of flu research. Part 2 will deal with modern attempts to “prove” OAS. The following review is intended as another supplement: A superficial examination of the initial debate over “Original Antigenic Sin,” such as it was, following the 1953 paper (reviewed in a
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
August 17: Refinements made to the description of “Receptor Destroying Enzyme.”
August 18: Cleared up the
Yewdell, JW. Santos, J. “Original Antigenic Sin: How Original? How Sinful?” Cold Spring Harb Perspect Med. 2021 May 3;11(5):a038786.
And also, by the retention of esoteric vaccine terminology that lacks in precision, eg. “immunized” instead of “injected with X.”
Additionally, with microscopes, it became possible to (eventually) observe such still-invisible entities at work upon bacteria - https://en.wikipedia.org/wiki/Frederick_Twort
Early recoveries of polio, based on passaging the virus into the spinal cords of rhesus monkeys, inadvertently generated a nerve-cell adapted strain. A strain suitable for researching the properties of the wild virus was not acquired until 1949, by Enders.
See “DNA Structure and Classic experiments, excerpt 1 | MIT 7.01SC Fundamentals of Biology.” Eric Lander (lecture). youtube.com
Shope, R. (1958.) “Influenza. History, Epidemiology, and Speculation.” Public Health Rep (1896). 1958 Feb; 73(2): 165–179.
(Regarding the myriad failed human challenge experiments during the Spanish Flu.)
Edward, DG. Elford, WJ. Laidlaw, PP. (1943.) “Studies on air-borne virus infections.” J Hyg (Lond). 1943 Jan; 43(1): 1–10.
Note that in Smith, W 1935, below, Smith makes an effort to test whether his minced-embryo-cultured flu virus is growing in tissue or fluid (akin to a bacteria).
Andrewes, CH. (1937.) “Influenza: Four Years' Progress.” Br Med J. 1937 Sep 11; 2(4001): 513–515.
Evidence that the Virus is the Cause of Influenza in Man
I will now summarize the evidence which convinces us that the virus we are studying is the primary cause of epidemics of influenza in man.
1.First, we have [in the overall course of research] recovered the virus from garglings of fifty-three patients with the symptoms of influenza obtained at times of rather widespread prevalence of the disease. During the recent epidemic virus was isolated from thirty-one out of forty-one uncomplicated cases tested. Virus has only been obtained during the first few days of infection and not during convalescence. We have failed to recover the virus from normal people, and we have usually failed also with garglings from sporadic cases or patients in localized outbreaks diagnosed as influenza but occurring in the absence of a widespread epidemic. [Emphasis added.]
Shope, R. (1934.) “The Infection Of Ferrets With Swine Influenza Virus.” J Exp Med. 1934 Jun 30; 60(1): 49–61.
Andrewes, CH. (1953.) “Epidemiology of influenza.” Bull World Health Organ. 1953; 8(5-6): 595–612.
And
Andrewes, CH. (1949a.) “Influenza in Perspective.” Edinb Med J. 1949 Aug; 56(8): 337–346.
There was quite a lot of influenza in Britain in the years up to 1847-48, when there was a big outbreak. After that, apart from a couple of minor flurries, the disease died down, not only in Britain but in Europe and North America, reaching a low ebb in the decade prior to 1889. There were, however, epidemics recorded in Russia in 1886 and 1887. These may be important, for the re-awakening of pandemic influenza seems to have started at Bokhara in Central Asia in May and June 1889.
Shope, R. (1936.) “The Incidence Of Neutralizing Antibodies For Swine Influenza Virus In The Sera Of Human Beings Of Different Ages.” J Exp Med. 1936 Apr 30; 63(5): 669–684.
Dr. J. S. Koen, an Inspector in the Division of Hog Cholera Control of the Bureau of Animal Industry, was the first to recognize the disease as being different from any previously encountered. He was so much impressed by the coincidental prevalence of human influenza and by the resemblance of the symptoms seen in man to those occurring at the time in hogs that he became convinced that the two were actually the same. He therefore gave the name of "flu" to this new disease of hogs. The opinion of Koen that "flu" represented an entirely new swine epizootic disease, not seen before 1918, was shared by many veterinary practitioners in the Middle West. Dimoch, in an exhaustive paper on the differential diagnosis of diseases of swine (12) presented in August, 1918, makes no mention of a disease of swine bearing any resemblance to influenza. It seems clear that swine influenza appeared in the Middle West as an epizootic disease for the first time, in recent years at least, during the late summer or early autumn of 1918
Shope, R. (1931.) “Swine Influenza I. Experimental Transmission And Pathology.” J Exp Med. 1931 Jul 31; 54(3): 349–359.
Eight strains of the virus have been established experimentally during three epizootic periods. The clinical disease induced by these eight strains has been in general the same although its severity and mortality have varied
Evans, DG. (1966.) “WILSON SMITH, 1897 - 1965.” Biogr Mem Fellows R Soc 12: 478–487
Smith, W. Andrewes, CH. Laidlaw, PP. “A Virus Obtained From Influenza Patients.” The Lancet. Volume 222, Issue 5732, 8 July 1933, Pages 66-68.
The MRC team reports 5 recoveries from human flu illnesses in their 1933 paper, but presumably the rest besides WS were not maintained.
Francis, T Jr. “Transmission of Influenza by a Filterable Virus.” Science. 16 Nov 1934 Vol 80, Issue 2081 pp. 457-459.
Francis, T Jr. Magill, TP. (1935.) “Immunological Studies With The Virus Of Influenza.” J Exp Med. 1935 Sep 30; 62(4): 505–516.
Magill’s first name is elusive. It is given here as Thomas.
Comments to this effect appear in multiple antigen-comparison papers after 1937.
Andrewes, CH. (1949b.) “Discussion: The Significance Of Strain Differences In Virus Prophylaxis.” Proc R Soc Med. 1949 Jul; 42(7): 517–522.
Now, the widespread occurrence of a new type of A seems to me to be surpassed in interest only by the virtual disappearance as an important cause of disease of the older A viruses related to PR8. Unless they stage a comeback, we may have to recordt hat, at any rate in Britain and America, they had their swan song in the autumn of 1943. The original strain of A, W.S., recovered in 1933 and perhaps 1934, has never been recognized since in the wild state. Were we at that time at the tail of a dominance of W.S. virus? Have we just been through a decade in which PR8-like viruses were dominant, and have we but lately entered on an era in which prevalent A viruses are related to the 1947 strains?
Francis, T Jr. (1953.) “Vaccination against influenza.” Bull World Health Organ. 1953; 8(5-6): 725–741.
Burnet, FM. (1935.) “Influenza Virus Isolated from an Australian Epidemic.” Medical Journal of Australia. 1935 Vol.2 pp.651-3
Shope, R. 1935. “The Infection Of Mice With Swine Influenza Virus.” J Exp Med. 1935 Sep 30; 62(4): 561–572.
Lately we have discovered that the failure of some strains to infect mice is only apparent; there is actually merely a failure to produce macroscopic lesions. If inoculated mice are killed on the third day after infection, and more mice are infected with an emulsion of their lungs, and so on, one will ultimately succeed in obtaining visible lesions in the lungs of the mice perhaps after three, perhaps only after six, passages.
(“Passage mor chiken” - local ferret.)
Smith, W. (1935.) “Cultivation of the Virus of Influenza.” Br J Exp Pathol. 1935 Dec; 16(6): 508–512.
(Smith was unhappy with his results for in-egg culturing, which were based on dropping filtered ferret lung juice on the chorioallantoic membrane.)
Burnet, FM. Line, PE. “A Genetic Approach to Variation in Influenza Viruses: 4. Recombination of Characters Between the Influenza Virus A Strain NWS and Strains of Different Serological Subtypes.” J Gen Microbiol. 1951 Feb;5(1):59-66.
The Melbourne strain adapted to mouse passage during 1935–6, but since 1940 maintained by storage of amniotic or allantoic fluid at the temperature of solid CO2
Note that a retrospective incorrectly claims that Burnet, having been adjacent to some of the first work in egg culturing with vaccinia virus, went directly to egg:
Wood, IJ. (1970.) “Burnet of Australia.” Postgraduate Medical Journal. (April 1970) 46,175-181. (pdf.)
The moniker “mouse-protection test” is applied by Smith in 1936 when publishing his method for a flu complement fixation test.
Smith, W. Andrewes, CH. (1938.) “Serological Races of Influenza Virus.” Br J Exp Pathol. 1938 Oct; 19(5): 293–314.
Citation pending.
This question was still debated on a case-by-case basis. For polio, there was still substantial push-back on the idea of subclinical infection leading to antibodies in absence of paralysis. See Harrison, J. Hudson, N. (1940.) “Study of the Serum-Neutralization Test in Poliomyelitis.” J Bacteriol. 1940 Apr; 39(4): 405–427.
Francis, T Jr. Magill, TP. (1936.) “The Incidence Of Neutralizing Antibodies For Human Influenza Virus In The Serum Of Human Individuals Of Different Ages.” J Exp Med. 1936 Apr 30; 63(5): 655–668.
Andrewes, Laidlaw, and Smith (3) in interpreting the significance of their neutralization experiments with human sera obtained in England, and the same strain of swine virus used in the present experi- ments (strain 15, Iowa, 1930) have guardedly suggested an explanation similar to that just outlined. They have qualified their interpretation by considering the possibility that the antibodies to swine virus in adult human sera may be non-specific in the sense that they represent past contact, not with that virus, but with some unknown related antigen.
Li, CP. Rivers, TM. “Cultivation Of Vaccine Virus.” J Exp Med. 1930 Sep 30; 52(4): 465–470.
As pus(“lymph”)-based smallpox vaccines were still in regular use in the decades afterward,* labs could independently derive their own egg-adapted vaccinia strain. For an example, see:
Oya, A. (1956.) “Growth Of Vaccinia And Variola Viruses In The One-Day-Old Fertile Hen's Egg.” Jpn J Med Sci Biol. 1956 Dec;9(6):293-302. (pdf.)
Vaccinia was therefor not only distinct from smallpox itself (due to over a century of animal and human passage), but still potentially an entire family of different viruses.
*Buchanan, G. Laidlaw, S. (1942.) “Vaccination in Glasgow Docks.” Br Med J. 1942 Oct 3; 2(4265): 394. (Note how “vaccination” here refers by default to the smallpox preparation, as no other injections were in common use in this era.)
Andrewes, CH. (1966.) “Twenty years' work on the common cold.” Proc R Soc Med. 1966 Jul; 59(7): 635–637.
Hirst, GK. (1942.) “The Quantitative Determination Of Influenza Virus And Antibodies By Means Of Red Cell Agglutination.” J Exp Med. 1942 Jan 1; 75(1): 49–64.
Laver, G. “Influenza virus surface glycoproteins, haemagglutinin and neuraminidase: a personal account.” Perspectives in Medical Virology. 7:31-47
Contemporary references to RDE from Vibrio cholerae describe its action as “destroying non-specific inhibitors” - things that might stop influenza HA from making red blood cells glom. This doesn’t seem plausible for an enzyme that binds sialic acid (the receptor for HA); my best guess is that RDE merely reduced the sensitivity of HI assays so that fewer false positives reduced the accuracy of measured inhibition.
In these, antibodies bound to an antigen will use up an artificially regulated loads of complement protein, rescuing antibody-bound sheep red blood cells from destruction. The first complement fixation test for flu virus was developed by Smith in 1936.
Stanley, WM. (1944.) “An Evaluation Of Methods For The Concentration And Purification Of Influenza Virus.” J Exp Med. 1944 Mar 1; 79(3): 255–266.
Stanley, WM. (1945.) “The Preparation And Properties Of Influenza Virus Vaccines Concentrated And Purified By Differential Centrifugation.” J Exp Med. 1945 Feb 1; 81(2): 193–218.
Cannell, JJ. et al. (2008.) “On the epidemiology of influenza.” Virol J. 2008; 5: 29.
This is an excellent essay, even if it inadvertently casts light on how little progress has been made since Andrewes’ time.
Oshinsky, David. Polio: An American Story. (Oxford University Press, 2005.) Ch. 6.
(Oshinsky, David. Ch. 6.)
















Wow, this is legitimately the stuff that would be relegated to science textbooks and used as for graduate level courses!
I will have to read this again a few times, but the most important factor in this article is the degree of laboratory procedures highlighted here. If we talk about people not wanting to know how the sausage is made, most people don't want to know how the study is conducted.
And so most people just want the punchline- tell us what this all means. In doing so, we miss over all of the effort that went into reaching the conclusion. Science is messy, science is dirty, and to be quite honest science is VERY boring. I have legit fallen asleep in lab a few times when it becomes too routine (or maybe that's just me...).
No one likes to hear the boring details because it's not mixing a few beakers to make something super colorful, or any of those other experiments conducted by pop scientists (if you have to preface your job with "pop", you're not a deep scientist).
So this is the truth of the matter. This is all of the nitty and gritty stuff that lead to the practices we have today. We can see all of the faults, but we always examine these practices through the lens of what is available at the time, and how they proceed. I'll say in the lab I used to work in I had people only a few years older than me tell me the crude lab practices they had which involved blotting blood onto special paper and just holding it up to the light and go "well, that's what that is!"
It's unfortunate that this is generally missed when we discuss science to the general public. It's not about what study was conducted, but all of the processes needed. I think that's why your posts may go over people's heads, and yet it's pivotal to understanding how you go through a study. There's a reason why neutralization assays don't tell us the whole story. There's a reason why PCR tests are done the way they are (and why they probably shouldn't be used for tests...).
Well, at this point I'm not sure where I was going, but thank you for such a deep dive that apparently only appears to go even deeper!
I've been thinking of writing about OAS because of my post from two weeks prior, and so maybe I can gain some inspiration from this post!
And what do you mean by "schizophrenic research habits"?! No such thing!
*Looks at 8 windows with 15 tabs each...
This is exactly the type of detail that is needed to correct the errors and groupthinks of the past, rather than basing future developments on their faulty science.
"Age of Enlighten-meh" 😅😅