Hundreds of millions of SARS-CoV-2 mRNA-LNP vaccine doses have already been administered to humans. However, we lack a comprehensive understanding of the immune effects of this platform.1
You don’t say.
(Correction notice in footnotes.2 Thanks to reader John (jc) Comeau, who writes at Iotecnotic, for pointing out the controversy over whether litters were sequential or serial.)
A Mousey Mystery
This post will review the recent preprint finding innate immune alteration from experimental LNP/+mRNA injections. Of course, it has already been covered by the recurrently avant-garde, poly-pseudonymed “Arkmedic.”3
Here is the paper.
The authors attempt to build on previous findings which identified the ionizable lipid component of the Lipid Nanoparticle packaging of the mRNA Covid vaccines as super-inflammatory, more-so common adjuvants.4 Given that the ionizable lipids have a half-life of weeks, does potential sustained inflammation leave a long-term impact on innate immune responses? Does it alter subsequent memory immunity responses, specifically by immune exhaustion? Is such an effect local to the injection site? And, naturally, does it mysteriously pass on to offspring.
They report, accurately or not:
LNP injection induces neutropenia; this is not surprising given that the prior study observed extreme recruitment of neutrophils, the immune system’s “first responders,” to the injection site. The first responders get all used up with… responding… to the super-inflammatory LNPs.
LNP injection depresses apparent T Cell recruitment by Langerhans cells — which are long-term sentinel dendritic cells — in the skin near the injection site.
LNP injection increases resistance to challenge with influenza, and decreases resistance to challenge with Candida albicans (i.e. yeast infection), suggesting promotion of anti-viral and suppression of anti-fungal innate immunity.
LNP injection decreases response to future LNP-mRNA-encoded antigens introduced to the same injection site or elsewhere, though not as much in the second case. There were indications of moving back toward baseline on the final week 8 timepoint, but full recovery was not observed at that point.
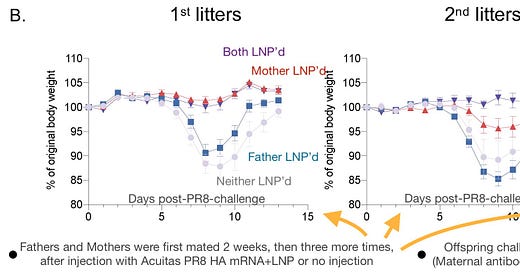
LNP injection with the mRNA for flu spike glycoprotein protected maternal offspring, and less clearly paternal offspring as well, for at least two successive litters. Dun, dun, dunnn. Except… here, there was no “control” LNP with either a different or no mRNA package, to establish that “flu protein memory” was actually being transferred. And so let’s start there, even though this results in reviewing the study backwards.
Findings Number 2: Adaptive Immunity in Offspring?
Mice were either injected with LNP-packaged mRNA for the flu spike glycoprotein (HA) or not. Injected and non-injected were mated in four combinations, creating four “teams” whose offspring were challenged with a sub-lethal dose of flu. Mating for the first litter took place two weeks after injection.
The authors do not seem to report the scheduling for the other litters. In fact, their description of the setup in this portion is so incomplete that I initially repeated the interpretation adopted by "Arkmedic" that there were multiple generations of mice born, mated, and flu-challenged, rather than multiple litters from the same parents. The reader should note that my current portrayal of the setup rests on a revised interpretation of the paper’s still-unclear text.5
Not only maternal injection, but paternal injection appeared to confer immunity (as in actual resistance to infection with) for the first and second litters.
Naturally, the authors reach straight for the p values to judge which trends count here. I prefer to trust my eyes over such superstitious divinations. For the “Father LNP’d” team, the performance vs. control in either the 1st or 2nd litter doesn’t strike me as impressive. However, the resilience of protection into the 2nd litter for the “Both LNP’d” team, vs. “Mother LNP’d,” is noteworthy.
Context: Innate, not Adaptive, Fetal Immune Reprogramming May Be Common
A number of recent studies have demonstrated that experiences during fetal development may “toughen up” offspring against later challenge, or predispose them to excessive inflammation. In “Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring,” for example, Lim, et al. summarize:
maternal infection affected the offspring intestinal milieu in a manner that enhanced TH17 cell reactivity toward the microbiota. Cross-fostering experiments demonstrated that the increased TH17 cells resulting from maternal infection was imprinted in utero. Among various inflammatory mediators, the cytokine interleukin-6 (IL-6) was significantly increased in the serum of dams infected with yopM. Injection of IL-6 alone to pregnant dams significantly increased TH17 cell numbers within the guts of offspring.6
Colonization of tissues by innate immune cells begins early in fetal development; and these immune cells — such as yolk-sac derived macrophages — may remain on the front lines until the first major immunological challenge or for a lifetime, depending on the tissue. Although the quote above is focused on the quantity of T Cells, it goes without saying that the character of these “pioneer” innate immune cells can be influenced by the maternal inflammatory milieu, the same way a boat full of colonists would probably, after settling, post more guards had a hail of arrows flown at them from the tree-line during their moorage.
As in the quote above, IL-6 injection alone is enough to promote militarization of the gut tissue. The earlier experiment by the team for today’s study found, not shockingly, that the hyper-inflammation promoted by LNP ionizable lipids induced IL-6 in mice:
We further found large amounts of interleukin-1β (IL-1β), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6, the signature cytokines of inflammatory responses
Given that the mice are mated two weeks after LNP injection, perhaps maternal inflammation is doing all the work here, especially given the questionable 1st-litter performance of team “Father LNP’d.” Or perhaps, at most, cytokines in the seminal fluid are just giving a tiny boost to the IL-6 exposure of fetal offspring.
Counter-argument: It’s still weird?
And yet, once again, the sustained performance of team “Both LNP’d” in the 2nd litter suggest that the father is playing a role in the enhanced protection of that may be more substantial than the incredibly sexy visual just offered above.
Still, I do not find myself ready to jump on board with “Arkmedic’s” conclusion that adaptive anti-flu immunity has somehow been inserted into the genome — either by incorporation of the mRNA code for the spike protein or other, unclear means.
Genetic modification of offspring is no easy feat, at least “on paper.” Our germ cells — sperm and eggs — unlike the rest of our cells, operate as a closed vault, preserving the “snapshot” of parentally-inhereted genes that were present in our earliest moments. Vaccine mRNA incorporation into random somatic cells anywhere in our body, if it is really happening at all, does not “incorporate” the same genes into any central “genome” — only those specific cells and any daughter cells. Paternal passage of such genetic modifications thus requires, first, that the mRNA gets into the specific sperm cell that fuses with the egg.
And so, since there are millions of sperm that vie for fusion with the egg, individual LNPs would have to deliver vaccine mRNA which would then have to successfully incorporate into most or all of these sperm cells, to make it remotely likely that this mysterious genetic gift would pass on to offspring.
What seems infinitely more plausible, is some poorly understood epigenetic marker — an autonomous self-modification to the sperm cells’ genes resulting from the LNP-induced inflammation — that then remains part of the next generation of (maternal or paternal) germ cells for this team.
And yet still, I find myself unconvinced. A short-term elevation in seminal fluid cytokines, which amplifies the embryonic inflammatory environment, seems more likely. (Note that I had originally posted an even more complex theory here when working under the impression that multiple generations of mice were being represented above.7)
Findings Number 1: Innate Immunity in the Recipient
As summarized above. LNP injection appears to lead to innate immune reprogramming, improving resistance against flu infection and diminishing resistance against a fungus.
Flu resistance is measured not just by weight loss but by PCR cycle-counts, with the non-injected mice showing lower cycles (and thus, higher viral loads). In contrast, the injected mice show higher levels of Candida in culture.
One extra note, regarding “LNP injection decreases response to future LNP-mRNA-encoded antigens introduced to the same injection site or elsewhere, though not as much in the second case.”
Interestingly, the depressive effect does not carry over to antigens delivered with different adjuvants. Despite high sensitization to LNP-induced inflammation, a robust response to new antigens could be produced by… inducing more inflammation. So why didn’t (second-injection) LNP produce the same effect?
Follow-up experiments showed lower fluorescent protein (GFP) production when an LNP-mRNA for GFP followed an LNP-mRNA for the flu spike glycoprotein (HA). Thus, it may simply be the case that there are fewer healthy cells left to turn into antigen-factories, leading to a reduction in antigen presentation. In the case of the traditional adjuvants, antigen was injected directly, and so there was no reduction in the payload.
Context: Non-Specific Effects Are Common
Altering and reprogramming innate immune responses via injection of foreign substances, i.e.“vaccines,” is not prima facie alarming. Innate immune reprogramming has been observed after lots of so-called “traditional” vaccines, and the adjuvants which dress them (emphasis added):
little attention has been given to the direct effects of adjuvants on innate immunity and early protection against infection. For instance, β-glucan (mainly encountered as a component of fungal cell walls that activates dectin-1) enhances [innate immune-mediated] resistance to acute infection with Toxoplasma and sepsis caused by Escherichia coli, respectively8
Beta glucans, which are just chains of glucose that feature funny, foot-to-shoulder linkages, are the molecules in the cell walls of fungi, including mushrooms. They have been used as an adjuvant - essentially a way to paint a target over other objects, either vaccine antigens or (experimentally) cancer. But it has been observed, as the quote above mentions, that they appear to also induce a non-specific impact on the innate immune system, heightening apparent resistance to protozoa and bacteria.
Some of this “reprogramming,” or “training,” may simply be an illusion. Alterations in measured innate immune response can be caused by prompting a change of the innate immune system guard. From the same review:
It has been shown that, following pulmonary insults, a reduction in yolk-sac-derived alveolar macrophages [which differentiate and colonize the lung during fetal development, i.e. while the body is still "booting up"9] is compensated for through the accumulation of [bone marrow]-derived macrophages [which are generated after the body has "booted up"] in the lung airways.
For example, infection of mice with gammaherpesviruses provided protection against allergic asthma, as it caused resident alveolar macrophages (AMs) to be replaced with [bone marrow]-derived AMs
So, some types of experiences which alter innate immune response are like drama between the current season’s cast-members in a reality TV show; some are like bringing in a new cast for the next season.
And, some are like changing the set itself:
Furthermore, the inflammatory site may also alter the functional capacity of the local stromal cells that induce trained immunity in residential innate immune cells.
Strikingly, following skin inflammation, epithelial stem cells maintain prolonged chromatin accessibility at key inflammatory genes [so, these files stay loaded up in the RAM, for ready access]. This feature expedites and heightens their response to subsequent stressors and potentially [definitely] influences stem cell cross-talk with trained immune cells
If last season was in a tropical island, and this one is in an office building that smells like mushrooms, the emotions of the cast (your innate immune cells) aren’t going to be the same.
All of these things will potentially measure as “innate immune reprogramming.”
LNP as an adjuvant
While the lipid nanoparticle, i.e. LNP, packaging of the mRNA Covid vaccines was billed as immune-evading, this team of authors, using the same packaging without any mRNA inside, previously found it to be extremely inflammatory. The key component seemed to be the ionizable lipids, the molecules which, like time-release membrane-destroyers, are meant to be neutral until they enter a cell inside of an endosome.
And so LNP, itself, seems to be an “adjuvant” after all, and so might induce the same kind of innate immune reprogramming observed for other adjuvants, regardless of how long it remains in the body. But it still remains true that innate immune reprogramming might not be a big deal compared to other concerns like, you know, having a bunch of ionizable lipids destroying membranes in your body for weeks on end.
Live Good, Dead Bad?
One possible rubric to consider, however, is whether productive “training” or detrimental “susceptibility” is more likely for certain categories of injections overall.
Christine Stabell Benn, a prolific researcher of non-specific vaccine effects (and one of the authors of the study quoted above), would say yes. Based mostly on mortality rates after vaccination in Africa (where more vaccines are still of the “live” variety), she advocates a model in which live vaccines lead to non-specific disease protection, inactivated vaccines lead to more non-specific disease susceptibility:
Based on these findings, Benn has become an occasional, cautious advocate for natural immunity, which of course makes her a rabid anti-vaxxer and menace to health. I will only note that this type of work, which is based on child mortality recorded from 1,000 feet above the scene on the ground, involving a far-from-exhaustive catalog of vaccine brands, is vulnerable to false discovery, and may not apply to all settings and demographics.
Mystery Status: Unconcluded
Qin, et al. is a provocative paper, but riddled with strange design choices — random, critical gaps in the control, which might render the results easier to sensationalize than they otherwise would have been. Specifically, mRNA for the flu glycoprotein (HA) was used in the offspring experiment, without checking whether any other kind of mRNA package would have the same effect. It’s not quite as egregious as the “spike protein goes into the nucleus” or “DNA integration” studies,10 but still enough to raise my alarms.
I do not mean to say that these studies are not discovering a valid truth. Just that it isn’t clear if they are.
This is always the important question, when evaluating science as a lay reader. Some scientists think they found something — but is the thing they think they found actually true? And if there are reasons why this might not be the case, interlocutors reporting the “findings” of the scientists should point those out.
An insider approach might consider “valid” any finding that is achieved with methodological precision, whether it is actually true, in reality, or not. All that matters is whether the work which produced that finding crossed the t’s and dotted the i’s.
It is in the former spirit that I report the limitations with studies which suggest novel genetic powers in the context of the Covid vaccines. There are reasons the things these studies claim to have found might not be true in reality. It is my hope that the reader perceives this as a service, and not any sort of effort on my part to impede the discovery that the Covid vaccines have turned all their recipients into mutant alien-virus-hybrids.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Qin, Z. et al. (2022.) “Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion.” biorxiv.org
The original version of this post described the study as having bred multiple generations of mice; instead, it appears that the same set of parents was bred multiple times. Further discussion in footnote 5.
“Dr Ah Kahn Syed.” “Who Owns Who?” (2022, September 1.) Arkmedic's blog.
Ndeupen, S. et al. (2021.) “The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory.” iScience. 2021 Dec 17; 24(12): 103479.
The authors specifically make it seem as if their interest was in the effect of injection on multiple generations of progeny.
A number of recent studies reported evidence for transmission of either trained immunity or tolerance across generations in mice
“Arkmedic” interpreted the setup the same way; and so did I, on first reading.
And yet, in discussing the results of their poorly-described multi-litter setup, they conclude (emphasis added):
Nevertheless, the overall protection levels fell across the board with later litters, suggesting that such heterologous effects do not persist for the entire life of an animal.
Since “later litters” reflect on the persistence of inheritable traits in “an” animal, litters 2 through 4 appear to be from the same parents.
Lim, A. et al. “Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring.” Science. 2021 Aug 27;373(6558):eabf3002.
The original, more complex theory:
An alternate explanation may again rest on cytokines in seminal fluid inducing a more “militarized” character of yolk-sac-derived innate immune cells, which is reinforced during the 1st litter’s lifetime. This reinforcement may not even result from the PR8 challenge, but from some environmental irritant. Either way, it results in a diminished repeat of the priming of the next litter’s innate immune cells, which finally fades out by the 4th litter.
Divangahi, M. et al. (2021.) “Trained immunity, tolerance, priming and differentiation: distinct immunological processes.” Nat Immunol. 2021 Jan; 22(1): 2–6.
Benn, who as mentioned is a co-author in this study, has made nonspecific effects of vaccines — does getting a measles vaccine protect children in Guinea-Bissau from random death? — a focus of her work. I would note that all of these findings raise my “replication crisis alarm,” and I think a lot of what is being observed is the limitations of randomization to remove bias, and the flaw in using death (a rare outcome) as a marker of overall impacts on health. Benn also co-authored the paper finding that adenovirus vaccines “reduce random death” in the sham Covid vaccine trials, a result clearly driven by an aberrant death rate in the placebo arm of the Janssen (J&J) trial. So, a grain-of-salt-approach for this whole subject is not a bad idea.
Hoeffel, G. Ginhoux, F. “Fetal monocytes and the origins of tissue-resident macrophages.” Cell Immunol. 2018 Aug;330:5-15.
See “Shaky DNA integration study.”
I was recently considering finally devoting a post to the spike-in-nucleus study. Upon revisit, it is too much of a “black box” to criticize productively.










if the synthetic genetic material is 'just' transiently out there reprogramming the jobs of few cells, how to explain super fast growing cancers of entire organs, 4 different types in one patient for example?
Reviews seem split between the 2nd, 3rd, and 4th litters being successive generations and, conversely, serial litters from the same 2 parents. I haven't read the study myself; you're sure it's the former?