"It's not actually just turtles, and also it's good that it's just turtles"
RE an attempted "Turtles" fact-check
Recently, Steve Kirsch posted an entertaining report on a failed, pro-vaccine attempt to debunk the criticism at the heart of Turtles All the Way Down, namely that vaccines largely are sent to market without comparison to true placebos, to allow identification of harms, rather than pseudo-placebos that potentially mirror harms and make identification impossible.
“What Turtles says is true, but ‘wrong’”
The attempted debunk in question is by a Professor of Law Dorit Rubenstein Reiss, who has contributed extensively to anti-disinformation site Skeptical Raptor and, for the purposes of evaluating her critique of Turtles’ critique of vaccine testing methodology, can be considered a qualified commentator despite her lack of biological background (after all, Turtles’ own authors also lack the same):

Reiss’s article nonetheless comes off as a rambling, disorganized and un-edited mess, and this is a consequence of the essential fact that she isn’t able to dispute the alleged “misinformation” repeated by Turtles at all. She can only affirm the claims over and over, with double-speak explanations for why she is not, in fact, affirming the claims, but refuting them. “It's not actually just turtles, and also it's good that it's just turtles.”
Consider that her article contains 8 instances of the word “wrong” (plus two in section headers), and every one of them self-evidently acts as a concession for a claim that cannot in fact be refuted, because it is based on a simple, rational description of reality. Her assertions of “wrong”-ness, followed by the first three of her arguments for the same:
Assertions:
“First, the book’s view of a “true placebo” is wrong and misleading”
“the book is wrong to suggest that decades of data collected on these vaccines are not good evidence about them”
“First, the book argues that the only valid control is saline (or sucrose) control. Second, while the book acknowledges that experts point out that when there is an approved vaccine it’s unethical to deprive a control group in the trial of it, the book then – based on an apparent misunderstanding – argues you should have a placebo group anyway. Both claims are wrong.”
“The book addresses three types of what they see as non-saline placebos. The use of another vaccine, and the use of the solution of the vaccine without the active ingredient, and argues that both distort the results. It’s wrong on both counts.”
“The book also argues that giving another vaccine to the children in the trial, even when some children are not given the trial vaccine, is not a valid test, and that, too, is wrong.”
“Finally, the most common criticism in the book was that children getting the trial vaccine were generally also given their routine childhood vaccine, which the authors think diluted the results. This criticism was made, for example, about Hib vaccines (58-59), polio vaccines (p. 60), pneumococcal vaccines (p. 61), and hepatitis A (pp. 62-63). This criticism is wrong in several ways.”
“This error – wrongly suggesting it’s appropriate to give a placebo in the context of an existing vaccine – addresses many of the claims the book makes about vaccine testing.”
“The book would like to dismiss evidence on vaccine safety besides randomized clinical trials. But that, too, is wrong.”
First three arguments:
“a clinical trial can be valid even if it does not use a saline solution as a placebo as a comparator, and in fact, sometimes using such a comparator is unethical.”
— In other words, Turtles’ notion of a “true placebo” is “wrong” because we just don’t want to use true placebos in vaccine development.
The “decades of data” collected on pre-modern vaccines are “good evidence” on “vaccine safety,” contra the argument in Turtles.
— They aren’t, and can’t be, evidence of anything, because they do not involve unvaccinated controls. In absence of controls, it is patently obvious that autoimmune (and other) conditions are increasing in children, and thus the “decades of data” that are available cannot argue anything other that childhood vaccines may cause harm.
Pseudo-placebos can be “valid” because the World Health Organization opines, in an advisory quoted by Reiss, that despite being a “methodological disadvantage” that renders it “difficult or impossible to assess fully the safety and reactogenicity of the trial vaccine,” pseudo-placebos may either “fulfill the ethical duty of beneficence and, sometimes, […] avoid giving an injection with an inert substance.”
— Neither of these rationales can render pseudo-placebos “valid” for safety evaluation, and the WHO expert panel as quoted by Reiss does not even claim otherwise (!).
And so it continues.
This is not to say that absolutely none of Reiss’ critiques of the rhetoric in Turtles are warranted, though as pointed out in Kirsch’s post she resorts to straw-manning seemingly wherever possible, and commits an important error regarding the compromised history of the current inactivated polio vaccine.
In particular, her argument that potential vaccine harms should be weighed against potential infection harms deserves mention for having merit in theory. But this argument isn’t germane or sufficient to waging the case that something is “misleading” about the fact that placebos are not used to facilitate measurement of what vaccine harms actually are.1 It literally does not matter whether Turtles addresses the question of net benefit, because that question is irrelevant to the systematic sabotage in modern vaccine development of any possibility of measuring the same.
Reiss may genuinely believe (in absence of evidence) that vaccine benefits so outweigh harms that it is “misleading” to argue that we should actually stop and measure harms before claiming vaccines are safe. But this is nothing but a circular argument. “Vaccines are safe, because they are good, and well, they wouldn’t be good if they weren’t safe, would they?”
I include mention of that tangent because Reiss places such emphasis on these types of lateral, big-picture concerns; but this emphasis itself is the problem with her “refutation.” The reason she must resort to “wholistic” arguments regarding tradeoffs and forced, “ethical” choices, is that Reiss isn’t actually contradicting the claim that true placebos are rarely used, she is just demanding the reader be happy about it.
Reiss isn’t actually contradicting the claim that true placebos are rarely used, she is just demanding the reader be happy about it.
Reiss can’t refute the central argument in Turtles, so she must simply brow-beat the reader into understanding why it is “wrong” to consider this a total invalidation of almost all vaccine safety claims, even though it is obviously just that.
Notably, Reiss frequently hyperlinks her claims to Paul Offit’s overview of vaccine development at the Children’s Hospital of Philadelphia website (his scientific propaganda outlet):

But if Turtles’ claims that vaccines do not establish safety with careful comparison to true, inert placebos is “wrong,” “misleading,” and “misinformation,” how can one explain Offit’s own survey of the field — is it not also “misleading,” etc.?
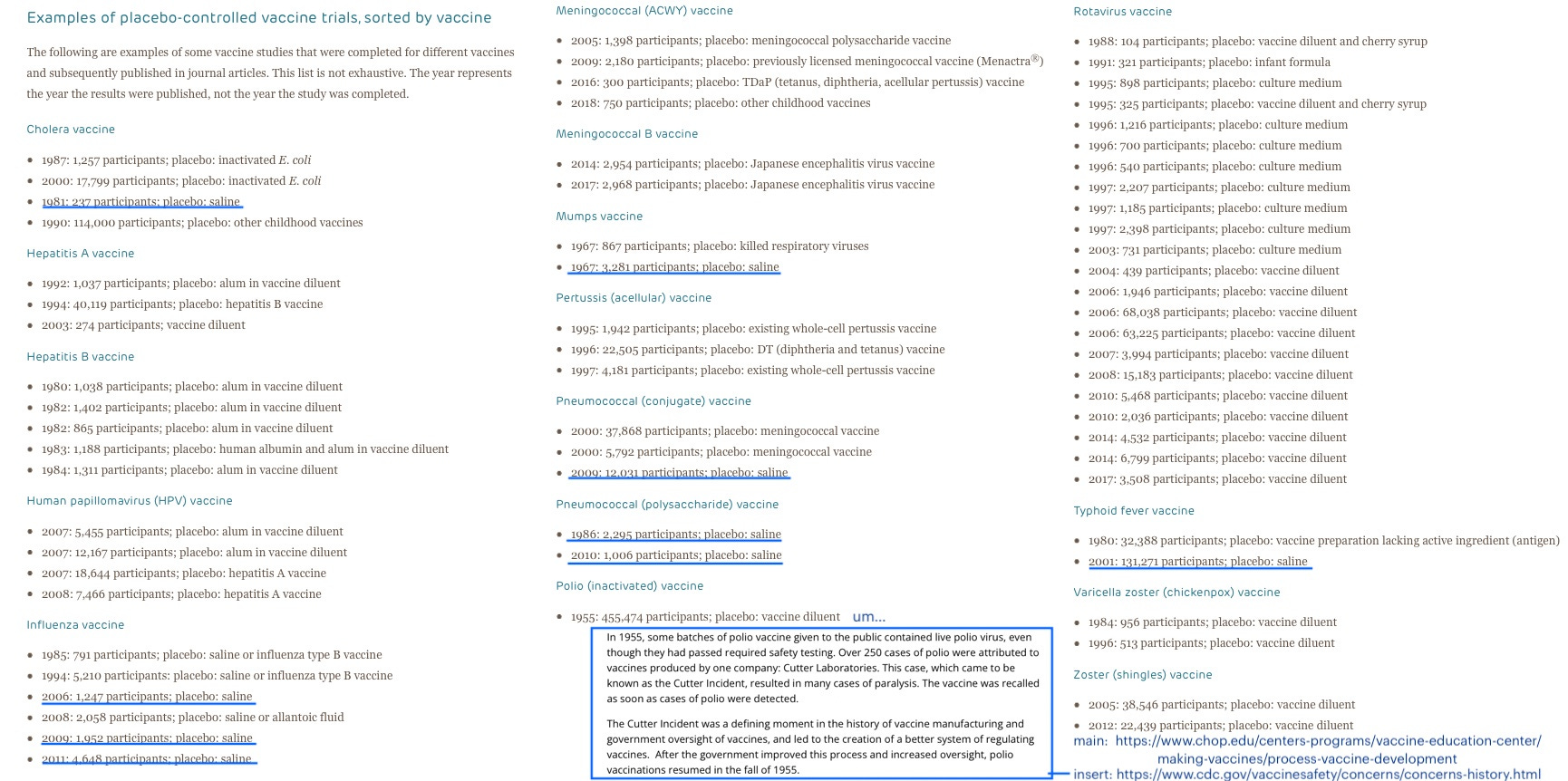
Polio vaccine, 1999: A galling case study in unmeasured safety
Offit’s inclusion of the Salk polio vaccine trial is quite the curiosity, given that it was a fiasco, and does not apply to the currently used versions of injections anyway.
In a staggering, historic farce rarely mentioned in the vaccine mythos, trial safety signals were mis-represented by Thomas Francis, Jr. (Salk’s patron who had inappropriately been put in charge of the entire trial operation and analysis), in his presentation given in advance of the rush licensure, with paralytic cases in vaccine recipients left unmentioned. This galling omission was made in spite of manufacturer quality concerns that had almost derailed the trial before it started (but which were instead waved off).2 And so almost immediately after licensure, it was unsurprisingly discovered that the one of the manufacturers had shipped live virus, resulting in hundreds of cases of polio and some deaths in the notorious “Cutter incident.”
Sabin had been among the scientific advisors at Ann Arbor who recommended licensing the Salk vaccine. But he had gone along reluctantly, as his correspondence makes clear. In a letter to William Workman, written several days before the Cutter incident became national news, Sabin complained that speed, not safety, had been the dominant concern. “During our hurried meeting at Ann Arbor on April 12,” he wrote, “we had to decide as to whether or not there was any evidence that the polio vaccine used in 1954 may by itself have been responsible for a certain number of cases of paralysis. Like the others, having had no time to examine the report, I was willing to accept the interpretation of Dr. Francis that there was no evidence….”
Now, after reading the various appendices and attachments, Sabin had his doubts. At least ten cases of paralytic polio had been reported in the first month of the 1954 trials among children who received the Salk vaccine. This was troubling. A few days after the Ann Arbor meeting, when Cutter hit the headlines, Sabin wasn’t surprised. He suspected there might be trouble and he thought he knew the cause.3
Not just the tragedy of Cutter, therefore, but the trial itself and the “Ann Arbor meeting” that led to endorsement of an obviously and openly rush-tested product (with $9 million worth of doses pre-purchased by O’Connor’s foundation ready to be injected the next day), should be emblems of shoddy vaccine development.
Yet this is hardly relevant to Offit’s survey, since the current licensed versions of inactivated polio vaccines are modern, “enhanced potency” reformulations with 2 to 8 times the concentration of antigen as Salk’s vaccine,4 which were never trialed with placebos to begin with. As pointed out in Kirsch’s post, this leads to a substantial error on Reiss’s part when she claims that Turtles “ignores” the Salk trial’s use of a placebo.
Third, the book has many inaccuracies – probably unintentionally; not knowing who the authors are, there is no basis to assume expertise. To give one example I will go back to when writing about polio vaccines the authors completely ignore the large placebo-based trials conducted for the Salk vaccine – whose formula is still the one used in the United States – in the 1950s.
Of course, it is obvious that “ignoring” the Salk trial does no favors to critics of vaccine safety; the trial revealed a glaring safety signal that was ignored, leading promptly to several deaths.
But Salk’s vaccine, its reputation never recovering from the Cutter incident (despite increased use after the first year), was discontinued in the US in favor of Sabin’s oral vaccine after 1963. Countries such as Sweden, Finland, and the Netherlands used their own formulations of inactivated, injected vaccines in the same decades — though this resulted in a series of small outbreaks in the early 1980s due to insufficient immunity, despite nearly universal vaccine uptake:
If we had used IPV, it is doubtful that we would have been as successful as the Dutch and the Scandinavians - and even those homogenous populations with their excellent vaccine coverage have had some outbreaks during recent years which, if extrapolated to our large population, would have meant many hundreds of paralytic cases and over a million carriers of wild virus.5
Regardless of these grim augeries, France switched to IPV in 1983, and Institut Mérieux makes the formulation reintroduced in the US a decade later to replace the Sabin oral vaccine, which is named “IPOL.”
The following overview of trials for IPOL is given in the FDA’s original approval document:6
Background Clinical Studies:
A clinical trial was carried out at Johns Hopkins University using a Poliovirus Vaccine Inactivated manufactured by Institut Merieux. This vaccine was manufactured using the same process as the current vaccine except the cell substrate was primary monkey kidney cells. After receiving doses at 2 and 4 months of age approximately 99% of the children had neutralizing antibodies to all three types of poliovirus. A significant increase in titers occurred after a third dose given at 18 months of age. There were 331 infants enrolled in the trial of whom 219 received three doses of inactivated poliovirus vaccine.
Clinical trials demonstrating Immunogenicitv and Safety:
Clinical trials with the vaccine made in Vero cells were carried out in infants at the State University of New York/Children’s Hospital, Buffalo, New York by clinical investigators Drs. H. Faden and P. Ogra and at Johns Hopkins University, Baltimore, Maryland by Drs. M. McBean and J. Modlin.7 In addition 30 adults were studied at the Buffalo study site.
These trials compared different combinations or schedules in infants using the Merieux Poliovirus Vaccine Inactivated and OPV. The schedules using IPV were a)IPV-IPV-IPV, b)IPV-OPV-OPV, c) IPV-IPV-OPV with doses given at 2, 4 and 12 months of age. Blood samples for antibody determinations were collected just prior to administration of each dose of vaccine and one month after the second and third doses of vaccine. […]
A total of approximately 400 doses of IPV were given in these studies. Safety was assessed in the Johns Hopkins study by a follow-up telephone' call with subjects at 24 hours and 2 and 3 days after each immunization to inquire about adverse experiences. Surveillance at Buffalo was limited to an interview during each immunization visit. No serious adverse experiences were reported at either study site. One adult complained of redness at the injection site.
There were no significant local or systemic reactions following injection of IPV. In the Johns Hopkins study, there were' 7% (6/86), 12% (8/65) and 4% (2/45) of children with temperatures over 100.6° F, following the first;' second and third doses respectively. Most of the children received DTP at the same time as IPV and therefore it was not possible to attribute reactions to a particular vaccine; however, such reactions were not significantly different than when DTP is given alone.
In sum, the inactivated polio vaccine given to nearly all children in the US since the turn of the century was only ever trialed on a few hundred infants, with no true placebo controls, and in conjunction with a never-placebo-trialed old vaccine — with minimal follow-up, confusing and changing manufacturing standards (the FDA trial descriptions do not easily line up with published descriptions), results not published for peer review until years after the trials took place, and with trial author Paul Albrecht’s signature on the FDA form.
By all appearances IPOL was approved as a delayed pet-project carried out when an author of a completely unimpressive, shoestring-budget trial happened to be in position at the FDA some years later. This caper in petite fraude is obviously, patently insufficient to determine anything about rare or long-term safety outcomes. It’s not even clear whether the early trial formulations bear any resemblance to those eventually marketed almost two decades later.
In sum, the inactivated polio vaccine given to nearly all children in the US since the turn of the century was only ever trialed on a few hundred infants, with no true placebo controls — with minimal follow-up, confusing and changing manufacturing standards, and results not published for peer review until years after the trials took place, and with trial author Paul Albrecht’s signature on the FDA form.
The self-dealing continues: When the CDC switched to recommending an all-IPV schedule for American children in 1999, John F Modlin, who in part conducted the tiny and poorly-documented trials over a decade before, was chairman on the advisory committee.

The published recommendations summarized the following evidence for safety of “IPV,” as obviously nothing was available for IPOL specifically:8
Vaccine Composition
Two IPV vaccine products are licensed in the United States,*** although only one (IPOL®) is both licensed and distributed in the United States. These products and their descriptions are as follows:
IPOL®. One dose (0.5 mL administered subcutaneously) consists of the sterile suspension of three types of poliovirus: type 1 (Mahoney), type 2 (MEF-1), and type 3 (Saukett). The viruses are grown on Vero cells, a continuous line of monkey kidney cells, by the microcarrier method. After concentration, purification, and formaldehyde inactivation, each dose of vaccine contains 40 D antigen units of type 1 poliovirus, 8 D antigen units of type 2, and 32 D antigen units of type 3. Each dose also contains 0.5% of 2-phenoxyethanol and up to 200 ppm of formaldehyde as preservatives, as well as trace amounts of neomycin, streptomycin, and polymyxin B used in vaccine production.
Safety
In countries relying on [non-enhanced] all-IPV schedules, no increased risk for serious adverse events has been observed. An extensive review by the Institute of Medicine (IOM) of adverse events associated with vaccination suggested that no serious adverse events have been associated with the use of IPV in these countries (61). Since expanded use of [primarily IPOL, enhanced] IPV in the United States in 1996, no serious adverse events have been linked to use of IPV (CDC, unpublished data, 1999).
Besides the resoundingly assuring “unpublished data” from the first few years of IPOL administration (given to infants in concert with other vaccines, naturally; and only comparable to the OPV, which whatever its virtues is known to carry a risk of paralysis), the only available safety record for modern enhanced-potency IPVs were the decades of real-world data from a handful of countries using non-enhanced formulations with no control groups.
The only available safety record for modern enhanced-potency IPVs were the decades of real-world data from a handful of countries using non-enhanced formulations with no control groups.
Available conclusions from these countries are summarized in the citation corresponding to (61). This document is the 1994 report from the Institute of Medicine titled “Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality (1994),” which was conducted and published in accordance with a clause from the 1986 National Childhood Vaccine Injury Act.9
Regarding the seldom-used, (non-enhanced) IPV, the document has a paucity of conclusions to offer:
There is no evidence bearing on a causal relation between IPV and transverse myelitis.
The evidence is inadequate to accept or reject a causal relation between IPV and GBS.
There is no evidence bearing on a causal relation between IPV and anaphylaxis.
There is no evidence bearing on a causal relation between IPV and thrombocytopenia.
The possible causal relation between polio vaccines and sudden infant death syndrome (SIDS) has rarely been studied. The evidence is inadequate to accept or reject a causal relation between polio vaccines and SIDS.
The evidence is inadequate to accept or reject a causal relation between [either oral or inactivated] polio vaccines and SIDS.
[No comment is offered for IPV and paralytic polio; it is merely asserted that “all problems” involved in the Cutter incident have been corrected.]
Overall, the non-enhanced IPV appeared more safe than the more widely-used attenuated, injected Measles vaccine, which is described in the same report as causing numerous instances of associated encephalitis cases. However, none of the “real-world data” from the handful of countries using non-enhanced IPV before 1983 satisfies the requirement of a control group (especially as these countries were all extremely high-uptake) to identify rare and unexpected harms; and the safety profile of the non-enhanced IPV cannot be assumed to proxy for modern formulations to begin with.
Conclusion: The safety of the modern IPV is completely unstudied
As the modern IPV was mass-authorized in absence of a placebo-controlled trial and with trial scales that do not meet modern Phase III standards, nothing about rare or long term adverse events can possibly be known. Granting that the OPV, which it replaced, was commonly found to cause paralysis at only a rate of about 1 per a million or so doses, there is no justification for having approved and switched to such a poorly-studied, novel replacement, which may not even provide lifetime immunity to the children born this century who have been the test subjects of the change.
The only apparent justification for Offit’s incorrect reference to the Salk trial, meanwhile, would be to avoid having to report that the current polio vaccine only involved a few hundred trial subjects, completely refuting his overall portrayal of vaccine development.
The only apparent justification for Offit’s incorrect reference to the Salk trial, meanwhile, would be to avoid having to report that the current polio vaccine only involved a few hundred trial subjects
Old vaccines are the mud in the laundry machine of modern “trials”
We may close with an observation on the fundamental hypocrisy of the argument that old vaccines should be used as “placebos” for new vaccines. Over and over in Reiss’s failed debunking, old vaccines and long-used vaccine additives (e.g. alum) are characterized as having “well-known” safety profiles.
But this just isn’t possible, because none of the old vaccines used as pseudo-placebos today were studied with trials using control groups.
This deficiency is the very reason that the Institute of Medicine was charged with reviewing the evidence for adverse events in the late 1980s. So in the very same era when vaccine trials began to use old vaccines as pseudo-placebos, the authors of the Adverse Events report wrote:
In the 1980s, however, a few concerned citizens in this country began to raise questions about the risks of vaccination. In fact, although the benefits to society were obvious, the risks to individual infants and children had not been well defined.
“The risks to individual infants and children [from all traditional vaccines] had not been well defined.”
Claiming that using controls without well-defined risks does not invalidate the ability of modern trials to demonstrate new vaccine risks is so patently spurious, that even the WHO refuses to do so. To quote, again:
A methodological disadvantage, however, is that trials using these types of placebos provide a less perfect control. It may be difficult or impossible to assess fully the safety and reactogenicity of the trial vaccine, although its efficacy can usually be assessed satisfactorily.10
And just as the CDC used two old, belatedly-published, under-scrutinized, and extremely small trials to back-door the enhanced IPV past modern standards, so too do old-vaccine-“placebos” back-door pre-modern blindspots into contemporary trial designs. If we could really know old vaccines are harmless based on real-world use alone, we wouldn’t have a need to impose randomized trial requirements on new vaccines to begin with. It would be obviously true either that, A.) Mid-20th Century vaccine designers were just wunderkind who made perfectly safe vaccines despite not using randomized trials using true placebos, or B.) We have no idea whether these old vaccines are safe, because they were not developed with randomized trials using true placebos.
Old-vaccine-“placebos” operate as a sort of inverse money-laundering scheme, in which the mud of poorly-studied injections is poured into the detergent slot while allegedly studying the next generation of injections, over and over, and whenever the process is criticized, the defense is an insulting volley of wishful thinking, moral blackmail, and outright idiocy.
But also, it may not matter.
And yet even given all the above, the “placebo” critique offered by Turtles may not in fact be the most important problem with vaccine safety. As long as society demands medical interventions for more than one or two childhood illnesses, it will never be realistically feasible to sort vaccine harms using randomized trials. Modern “placebo” subjects for future vaccines, even if given true placebos in the trial context, are still likely to receive other, previously-authorized vaccines, and this will confound any attempt to measure harms from any individual vaccine.
Absent “human experimentation” as traditionally envisioned (in which a select number of individuals are subjected to extreme conditions, but in a controlled context), laissez faire human experimentation (in which all of humanity is subject to extreme conditions, in an uncontrolled context) will forever be the only way vaccination can continue as a social practice. “Science,” in the form of studies and trials, does not have the power to detect whether vaccines are harming us. And perhaps it is in offering an illusion of an alternative that Turtles is correct, but “wrong.”
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
It is further the case that reduced and induced harms are unlikely to be measurable absent long-term trials with true placebos in most cases. An exception that demonstrates the problem is Measles, for which the attenuated vaccine could authentically replicate natural infection (which involves viral spread in the bloodstream); this made it easy to compare certain adverse events side-by-side, particularly encephalitis, with little question as to whether the vaccine was plausibly associated, but strong evidence that infection was associated with an even higher rate. Further, if attenuated Measles vaccination (like natural infection) prevents later instance of Measles infection of the bloodstream, it plausibly prevents all infection-associated encephalitis (and etc.); though proof of such true immunity is weak.
Whereas vaccines targeted at agents that produce rarer and thus less clearly measurable harms (for example, SARS-CoV-2 in children), cannot be expected to demonstrate a measurable net benefit; and the vaccine does not by necessity prevent a particular stage of natural infection, since in children infection is local and not systemic — it could plausibly be the case that infection-associated adverse outcomes happen nearly or just as frequently despite vaccination.
Therefore, in practice (rather than in theory) prevented infection harms should not be assumed to grant most childhood vaccines a pass for any amount of (intentionally unmeasured) harms.
Oshinsky, David. Polio: An American Story. (Oxford University Press, 2005.) Ch. 12.
(Oshinsky, David. Ch. 13.)
Modlin, JF. et al. “Humoral and Mucosal Immunity in Infants Induced by Three Sequential Inactivated Poliovirus Vaccine-Live Attenuated Oral Poliovirus Vaccine Immunization Schedules.” J Infect Dis. 1997 Feb;175 Suppl 1:S228-34.
The 2 to 8 times figure cites a 1986 paper by RH Bernier which is not available online.
Melnick, JL. “Vaccination against poliomyelitis: present possibilities and future prospects.” Am J Public Health. 1988 March; 78(3): 304–305.
Faden H, Modlin JF, Thoms ML, McBean AM, Femdon MB, Ogra PL. “Comparative evaluation of immunization with live attenuated and enhanced potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses.” J Infect Dis. 1990; 162:1291-7.
The Johns Hopkins trial appears to also have been published two years earlier (which is still 5 years after completion):
McBean AM, Thoms ML, Albrecht P, Cuthie JC, Bernier R. “Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines.” J Epidemiol. 1988 Sep;128(3):615-28.
Prevots, DR. et al. (2000.) “Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP).” MMWR Recomm Rep. 2000 May 9;49(RR-5):1-22; quiz CE1-7.









She focused on Placebo as its the weakest argument, although a valid one , among all those presented in the Turtle Book regarding safety.
As we saw with the COVID VACCINES using a true placebo does not guarantee detection of an unsafe vaccine since those running the trial have perfected not finding problem
And even if a particular vaccine were found to be safe on its own, it could still contribute to a cumulative adverse effect on children who receive all the recommended vaccines.
A clinical trial of a new aluminum-containing vaccine cannot identify long-term health effects caused by the gradual accumulation of aluminum in an infant’s body.
Clinical trials of vaccines typically do not report chronic syndromes and diseases, such as autism, attention deficit hyperactivity disorder (ADHD), diabetes, or cancer. These conditions develop over a period of months or years, and consequently researchers tend not to associate them with the tested vaccine even if they are diagnosed during the time frame of the clinical trial.
The lack of safety testing of the cumulative effect of vaccines during their approval process, as well as the inherent limitations of adverse event reporting systems, has not attracted the attention of the FDA or CDC.
In order to evaluate the safety of the entire vaccination program, as well as the impact of vaccines on adverse health conditions that develop in the medium and long terms, one must conduct studies comparing the health of subjects who were fully vaccinated with the health of those who were not.
I know this is tangential to your post. I was born in 1954 when, presumably, the polio freakout was in full swing. I did have an uncle who had to wear leg braces so we were definitely aware of it.
My parents are gone and there's no one else I can ask who might remember. I wonder if I got the Salk vaccination (as you say, released in 1954) and then later on the Sabin OPV. Even at the tender age of six or seven, I remember much talk about Salk versus Sabin. I do distinctly remember them lining up every kid in my school, to get the sugar cube with the pink stain on it. It was memorable because sweets were not as pervasive then (excitement!) and sugar cubes were a novelty.
We all got measles and chickenpox as a matter of course. I never heard of anybody who died. Somehow everyone in my family escaped the mumps.
I can't help wondering if the increase in SIDS and ADHD has been at least partly a result of the increase in infant vaccinations. Seems reasonable.