Hahn vs. Trump
Comparing the new "Trump rushed it" Covid vaccine narrative to the Hahn interview transcript.
Under Pressure
Media reporting of the House crisis committee’s latest triptych of charges1 against the Trump administration’s response to the virus seems like the first move in a coming campaign to disavow the Covid vaccines.
This is probably not the case; the temptation to perceive it as such is likely an accident of timing: The committee’s report and subsequent media coverage arrive at the same time as media reports of persistent “unexplained” excess deaths appear in multiple countries, and anecdotally-recorded sudden deaths sky-rocket - to such a degree that instead of the weather, occupation of the latest victims can be used to mark the days. Monday and Thursday were jazz instrumentalist (51 and 39), Tuesday mountain biker (37), etc.2
Still, I found the story of just what happened between Trump and the FDA intriguing enough that I spent this day comparing the House report with the transcript of the subcommittee’s interview with former commissioner Hahn in January of this year.
If you anticipate life-changing insights and wisdom from this post, why not “tip first, ask questions later”?
My questions / takeaways:
Was there any actual delay for “safety” in the FDA process?
Yes and no. Hahn and colleagues at the FDA did set the guidance for the manufacturers to require half of participants to reach at least the 60-day mark after the injection course before submitting their final application data.
This, it should be clear, was entirely ad hoc [see transcript in Supplemental, below]. The FDA could have picked any adverse event monitoring time-threshold they wanted, including none at all.
However, in the Pfizer trial at least, the 60-day milestone had all-but passed by the time enough infection outcomes were reported for the interim (November 4) and final (November 14) efficacy analysis benchmarks (the timeline described here previously3).
(If anything, it’s a bit suspicious how both the efficacy and safety benchmarks were met just after election day.)
What was the justification for Hahn and the FDA’s delay?
Scientific tropes about time-frames for previous identified vaccine toxicities. More specifics in the Supplemental section below.
How does this “safety” measure align with the FDA’s overall approach?
Farcically.
The FDA never had any power to enforce accurate reporting of adverse events by manufacturers to begin with. Did the numbers they received match reality? How could they check?
Maybe by responding to warning signs. And yet, the FDA failed to act on Brook Jackson’s reports in late September of staggering oversights at a Pfizer vendor.
The next morning, 25 September 2020, Jackson called the FDA to warn about unsound practices in Pfizer’s clinical trial at Ventavia. She then reported her concerns in an email to the agency. In the afternoon Ventavia fired Jackson—deemed “not a good fit,” according to her separation letter.4
And while the FDA’s Emergency Use Authorization-enabled “rolling review” overall design was logical (even if I would still deny that there was ever a true “emergency”), the accelerated timeline for processing the submitted application guaranteed that the end result was nothing more than a perfunctory sign-off. There was no way for the FDA to fish out whatever safety signals that the manufacturers had kept hidden or subtle (though at the same time, no excuse for missing the glaring issues with lopsided “Covid-like illness” and exclusions in the intervention arm5). From Hahn's testimony (emphasis added):6
HAHN
We refer to it as rolling reviews. Someone would say we want to submit an [EUA] application, we deemed it a priority [as opposed to normal first-come-first-serve scheduling], and we would go back and forth with the developer [as they were compiling the application] and say we need these data. They would send preliminary data. We would review that before a final package was submitted [often resulting in next-day decisions when all FDA feedback preceded the client application, as in the case of many PCR tests].
[…]
DEMOCRATIC COUNSEL
Do you agree [with detractors from Trump’s circle] that there was bureaucratic slowness?
HAHN
Well, I do agree that [the FDA], HHS [which encompasses the FDA], etc., that we all could have done better from a process point of view. I mean, it has to undergo legal, ethics, etc., review [in other words, it took extra time to identify all the legal corners that could be cut in an EUA context].
I do not agree on the scientific side, because I — if you look at vaccines, it took us three weeks to review a competed application that was tens of thousands of pages long, and that would normally take months.
So, was Trump the driver of what the FDA did rush WRT the Covid vaccines?
No. As Hahn reports, the FDA understood its mission to be to do everything possible to accelerate deployment of therapeutics and vaccines. Thus, all admonitions from Trump’s circle to “hurry” were delivered to an organization that was already hurrying.
DEMOCRATIC COUNSEL
Moving on, are you aware whether President Trump or any member of the administration sought to speed up the review or approval of any coronavirus treatment?
HAHN
You’re asking did President Trump or anyone in the adminstration attempt to speed up. So the answer is, in general, the Trump administration, the President on down the administration was all about trying to get speedy approval of medical products for Covid. So in a broad sense, the answer is yes, because in that — the President was all about that, the speed part of it.
DC
Did this — where you ever concerned by the desire to speed up the review and approval of therapeutics?
HAHN
That part of it, no, because — I mean, we were [pretending to be in] in a public health emergency and it was totally appropriate to ask the question, what can you do to speed this up to get lifesaving treatments, vaccines, [experimental genetic constructs] etc., into the hands of people?
In that case, what was the admin’s response, after the September submission for clearance, to the proposed 60-day delay?
Confusion, hostility. Secretary Azar or intermediaries at the HHS, after soft inquiries, honed in on the 60-day threshold:
There were questions about whether the 60-day meeting follow-up in particular was appropriate given the [widely-assumed] urgency of the situation
After multiple restatements of the science-trope-centric basis of the 60-day threshold, the HHS forwarded the FDA’s guidelines to the White House for final sign-off. At this point, the manufacturers had already been keyed in on the FDA’s standard per the open, “rolling review” communication channels, and Trump’s circle perceived, accurately or not, a direct attempt by Hahn to sabotage 2020 election results by postponing the EUAs. Unproductive pushback ensued, with the White House relenting “several weeks” later.
HAHN
It went to the White House. There were objections about it and there were suggestions made about adding additional language. Some of it was around availability of the vaccines and distribution, which isn’t in our bailiwick, and others were really pushback about the issue of the 60 days.
[…]
HAHN
I mentioned one was a distribution change. There might have been others as well. But just to be really clear about this, I felt very strongly about the fact that our scientists had created this guidance, I totally supported the science and the clinical data behind it, and I objected to any suggestion that it be changed because I really felt that the state needed to stay in the scientific and clinical domain, and I also felt any changes would obviously be reported and would further reduce vaccine confidence.
DC
So what happened next?
HAHN
In early October, Mr. Meadows called me and told me that it had been approved by the White House and we could go forward. And we subsequently published the guidance.
Once again, the net impact of the “safety” delay was nil, since the Pfizer efficacy benchmarks were not met until post-election. Nor did Trump’s stonewalling of the delay exacerbate or accelerate data submission to the FDA, thanks to the rolling review design - the whole thing can be imagined as a gaggle of track coaches arguing about where to set the finish line after the starting gun has already been fired, but before the eventually agreed-upon threshold is crossed: It was always ahead of the runners’ current position, anyway.
At the same time, the perspective of the admin — “Wait, why are any experts suddenly worrying about safety now, with the election just around the corner?” — is entirely sympathetic.
What, if anything, was the influence of prior conflicts in exacerbating the White House/FDA vaccine-EUA conflict?
Probably, a lot. The majority of the subcommittee’s inquiry and Hahn’s testimony is devoted to these conflicts.
HCQ
First, regarding hydroxychloroquine, Hahn and the FDA’s revocation of the EUA is seen as a deliberate attempt to side with the media and discredit the administration’s promotion of the drug to mitigate severe outcomes.7 In the end, this seems to be an instance where Hahn, conditioned to distrust Phase 2 studies from his experience as a cancer doctor, was determined to call the wrong shot as soon as the published evidence granted him an excuse to do so. It is difficult to distinguish his ingrained reverence for RCTs ("the gold standard") from some nefarious outside influence. And thus at the first RCT to poison results with post-hospitalization dosages of HCQ, from Brazil, the trigger was pulled.
August Press Conference Awkwardness
Secondly, immediately preceding the FDA “safety” requirement submission to the HHS / White House, was the Convalescent Plasma Fiasco.
Here, Hahn seems to have been “emotionally pumped-up” into relaying a relative risk reduction as an absolute risk reduction.

The “survival-increaser” in question is collected plasma from SARS-CoV-2 convalescents, which naturally contains protective antibodies that reduce viremia. Despite low interest from Pharma in this century-old technology, the FDA was eventually able to enroll the Mayo clinic in an expanded access trial. Results, measured by relative risk, were impressive - a 35% mortality reduction. This is basically half as good at preventing death as the experimental vaccines.
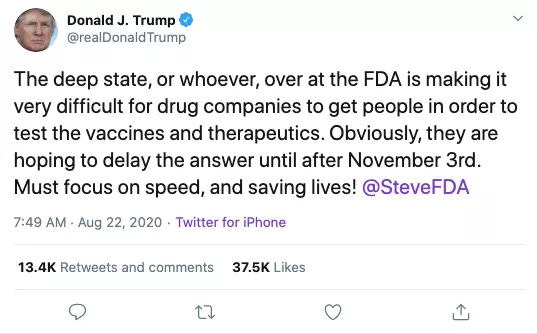
An NIH complaint, however, delayed the inevitable FDA authorization of the treatment, with the result being that Trump launched into a twitter-tirade against the “deep state, or whoever,” just as the FDA was completing the complaint-prompted extra analysis.
From the perspective of Hahn:
DEMOCRATIC CONSUL
On August 23rd, 2020, FDA granted the emergency use authorization for convalescent plasma. Who made the ultimate decision to authorize the EUA?
HAHN
Dr. Marks and the review team for Biological Evaluation Research, CBER.
DC
When was that decision reached?
HAHN
That weekend.
DC
Do you recall that day?
HAHN
Dr. Marks had communicated to me that maybe even the week before that they [CBER] had come to this conclusion. They were reanalyzing data [according to the NIH complaint’s standards] as it came in just to have as complete of an accurate picture as possible. But this decision to proceed had mostly been made pending this additional review, and it was coming in on a regular basis.
So I remember talking to Dr. Marks on Friday and Saturday [August 22, before Trump’s tweet and phone call], and it had been pretty much decided at that point that the EUA be issued.
On the same day as “pretty much decided,” Trump fired off the above tweet which tagged Hahn and alleged that the Deep State, or whoever was delaying new therapeutics and vaccines. Hahn followed up with Trump in a phone call to explain that the FDA was not imposing any specific extra requirements for either.
The in-progress EUA for convalescent plasma went live the next morning, prompting a press conference. Hahn characterized the 35% mortality reduction in absolute, rather than relative terms, promising 35 saved “Covid-19” patients for every 100. He was immediately burned at the stake by social media scientists.

After his session on the twitter torture rack, Hahn apologized for his extra-overtly-misleading portrayal of the benefit of convalescent plasma, and fired the recently Trump-appointed FDA communication coordinator who seems to have been instrumental in pumping him up to make his on-air comments.
What is amusing about this farce, is that doctors and pharma reps routinely mean for the lay public to misunderstand relative reductions and absolute reductions. It seems entirely plausible that Hahn, psyched-up in advance of the press conference, did not realize he was getting the math “wrong” here precisely because, in terms of impression the lay audience is meant to receive, the math was exactly “right.”
Nonetheless, this episode of reputation-damage clearly could have played a role in motivating Hahn to hold the line when it came to the FDA’s perfunctory 2-month safety threshold. Neither he nor the FDA had much “independent scientist” credit left to spend in favor of promoting new interventions, alleged “Pandemic Emergency” be damned. Let them eat spike; the Agency must prevail.
What has driven most reportage about the Trump circle’s “interference”?
Primarily, inability of politicians and the media to leverage a few brain cells to apprehend the nuances implied by “rolling review.”
Hahn’s testimony is misunderstood and mischaracterized based on the idea that any challenge to the FDA team’s thresholds implicitly rushed or delayed approval. This is nonsense, as the rolling review design builds in the rush, and efficacy thresholds were achieved after safety thresholds, making delay de facto impossible.
In the end, Trump neither accelerated nor delayed submission of the EUA applications, given the redundant efficacy thresholds; and the responsibility for ignoring whistle-blowers and skimming over the paperwork rests with the FDA.
DC
Several weeks? What were the consequences, if any, of [the White House’s “several week”] delay [of approving the 60-day window]?
HAHN
I don’t think that there were [really, like, 1-1.5 months is less than 60 days? what?] — I mean, let’s just put it this way. There weren’t any consequences from the clinical development point of view in the way the studies were conducted, because we had already communicated [per the rolling review protocol already explained 10 different times] that [a 60-day safety threshold] was something that we were interested in seeing. I think it was unfortunate that there was a lot of press around this [implying that manufacturers could only have been informed of the non-White-House-approved safety threshold via some workaround “leak,” rather than the open EUA comm lines]
Supplemental: Hahn’s testimony RE 60-day delay
DEMOCRATIC COUNSEL
In September 2020, it was widely reported that FDA was working on new guidance that would be followed before authorizing a vaccine-related EUA. How did that come about?
HAHN
[Name of the Democratic Counsel], if it's the guidance that I think you’re referring to, that was August, September, October.
DC
Okay. Then when did that process start?
HAHN
In the summer.
DC
In the summer? And why?
HAHN
So we felt strongly that we needed to provide guidance to industry about what actually would be required, provide as much transparency about that to industry, so we started off with a vaccine [meaning development] guidance that was issued, I believe, the end of June, early July. Then we provided additional guidance about what criteria we would be looking at for an actual EUA. So the first guidance was about, here’s how to develop the vaccine. The second guidance was about these are the data we need to see to feel comfortable, again, potentially providing an authorization. No promise, but this is what we needed to see.
DC
Who led this effort to develop this new guideline?
HAHN
We did, at the FDA.
DC
Was there one person specifically that was leading the effort?
HAHN
Well, the vaccine division under Dr. Marks and CBER, Center for Biological Evaluation Research.
DC
You said that the purpose of the guidance was to provide clarity or transparency to manufacturers. What specific criteria was discussed that would be put into the second piece of the guidance about what --
HAHN
We would need to see --
DC
-- you would need to see? Exactly.
HAHN
So we would need to see data from at least one adequately powered randomized trial on the efficacy side. And then with respect to toxicity, we wanted to see the median follow-up of participants in the trial had completed at least 60 days of follow-up.
DC
Were those the provisions that were ultimately incorporated into the guidance that was issued?
HAHN
Yes.
DC
Were additional requirements discussed, but ultimately not put forward in the final guidance?
HAHN
Discussed by whom?
DC
By anyone at FDA.
HAHN
I don't know, actually. I mean, so the process at FDA, we would discuss the whole range of things. I mean, as you can imagine, it's a very complicated process, and we would look in the literature, we would look at our own experience. So I guess my answer to that is, yes, we probably discussed a lot of things, but it came down to this as the most appropriate and pragmatic way to assess the vaccines. Balancing, again, with speed, with making sure we got the decision right.
DC
How did the decision come to be made to require 60 days of evaluation after the second dose?
HAHN
Well, we had looked at the literature and our own experience with when toxicities would manifest themselves. Just to put it in perspective, with the normal time of the vaccine, you're going to have potential toxicities develop well after the data's submitted. So even under normal circumstances, there's practically no medical product that you can 100 percent guarantee in the real-world setting won't have some unexpected toxicity. So the question is, how do you stratify the risk versus the benefit? In this case, we looked at the literature, saw where the overwhelming majority of toxicities were seen except for the very rare toxicities and came to the conclusion that 60 days was an appropriate measure for that.8 Now, a part of that calculation was if you could predict an efficacy floor, which we did, of 50 percent, how many lives would be saved if it was in fact efficacious and deemed safe at 60 versus 90 versus 120? And it was very clear from our analysis in that risk-based approach that 60 was a reasonable place to sit.
DC
Were you aware of whether higher standards or lower standards were — fewer days, more days — were proposed in the medical literature or by anyone at FDA?
HAHN
I don’t know about at FDA, but I am aware that the WHO stated publicly and published that they would look at 90 days.
DC
Why did the determination come to be made that 60 was better than 90?
HAHN
Again, we looked at our own experience internally as well as the literature as to when toxicities were seen. We felt — so, [name of DC], it’s all an issue of how many more lives could be saved if we did it 30 days earlier versus what are the risks associated with this? And this is a core FDA responsibility is to assess the risk-benefit ratio.
DC
You said this process started in August. When was the guidance ultimately released?
HAHN
October, early October. You’re talking about the guidance on the data we’d need to see for EUA?
DC
Correct.
HAHN
Yeah.
DC
Can you take me through the process of how this started in August and why it took ultimately until October for it to be released?
HAHN
So the whole initial guidance started in April, May, issued in late June, July as we went through the process. Now, I think it’s important to remember that although it certainly seems like it took a long time, we were communicating on a regular basis with industry about what our expectations were. So — and the trials could always be modified based upon what we ultimately came up with. But we came to this conclusion in August, September, and then we went through the normal process of having it reviewed and approved through the normal mechanism of HHS and then to the White House.
DC
When did you ultimately send it up for approval to HHS and the White House?
HAHN
I don’t remember exactly. My guess, it would be September.
DC
What was the reaction?
HAHN
Initially, there were questions about it, we provided clarification, and it looked like it was going to be allowed to move forward.
DC
What were the questions?
HAHN
Very similar to your questions: Why do we pick the 60 days? Why the median follow-up? What is that based upon? Scrutiny over the scientific and clinical rationale for what we were seeing.
DC
Did the FDA receive any pushback?
HAHN
Yes.
DC
Of what? What happened?
HAHN
There were questions about whether the 60-day meeting follow-up in particular was appropriate given the urgency of the situation.
DC
Who raised that concern?
HAHN
Questions were raised at HHS as well as at the White House.
DC
Who at HHS?
HAHN
Some of it emanated from the Secretary’s office. […] I had a conversation with Secretary Azar, the team did, Paul Mango in the Secretary's office, I believe Brian Harrison was involved as well, the Secretary's chief of staff. And it was around the timeline, scientific rationale, all the issues that we had just discussed. […]
DC
Did they ask the FDA to make changes to the time period?
HAHN
Not initially.
DC
What happened?
HAHN
It went to the White House. There were objections about it and there were suggestions made about adding additional language. Some of it was around availability of the vaccines and distribution, which isn’t in our bailiwick, and others were really pushback about the issue of the 60 days.
DC
So I want to go through that in a little more detail. You said that there were objections at the White House about it. Who objected to that?
[Meadows and Mango are named, this part draws out…]
DC
What changes?
HAHN
I mentioned one was a distribution change. There might have been others as well. But just to be really clear about this, I felt very strongly about the fact that our scientists had created this guidance, I totally supported the science and the clinical data behind it, and I objected to any suggestion that it be changed because I really felt that the state needed to stay in the scientific and clinical domain, and I also felt any changes would obviously be reported and would further reduce vaccine confidence.
DC
So what happened next?
HAHN
In early October, Mr. Meadows called me and told me that it had been approved by the White House and we could go forward. And we subsequently published the guidance.
DC
Had a copy of the guidance previously been provided to anyone outside of the Trump administration?
HAHN
Yes.
DC
Who?
HAHN
To industry.
DC
Had the White House approved providing the guidance to industry before? [This is a stupid question; Hahn has already explained rolling review numerous times and now he must explain it again.]
HAHN
So we didn’t call it guidance at the time. We had communication with them as they were constructing their Phase 3 trials. So we communicated that outside of the formal guidance. Which happens a lot informally. It wouldn’t typically be something that we would communicate or need approval for.
DC
So when were those — when was that happening? When were those discussions or when was it provided to industry?
HAHN
My understanding from Dr. Marks is that happened in the summer.
DC
And so are you saying it was not uncommon to have discussions with industry about standards that might not ultimately come to pass? [Totally implied by his numerous descriptions of rolling review; they are a back-and-forth when benchmarks are still flexible.]
HAHN
No. Standards that wouldn’t necessarily be put into a formulated formal guidance. You know, these informal conversations occur with developers all the time. This is the current clinical situation, this is what we’re looking at, this is our experience with your drug, vaccine, you name it. This is what we’re recommending to you that you have as part of your package. Those discussions occur at levels of the agency every day.
DC
And so how does the interplay work if it’s not — if it’s discussed with industry but not formally —
HAHN
A guidance?
DC
— formally a guidance?
HAHN
That’s what we call TA or technical assistance. Industry in general tends to follow it because you’re talking to the reviewers who are going to look at your application.
DC
How long did it take between the guidance being raised to HHS and the White House and it ultimately being approved?
HAHN
[Name of DC], it would have to be a guess, but several weeks.
DC
Several weeks? What were the consequences, if any, of that delay?
HAHN
I don’t think that there were — I mean, let’s just put it this way. There weren’t any consequences from the clinical development point of view in the way the studies were conducted, because we had already communicated that was something that we were interested in seeing. I think it was unfortunate that there was a lot of press around this. And, again, the whole environment context contributed to a lack of vaccine confidence.
If only.
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Chinen, Nate. “Lauded trumpeter and composer jaimie branch dies at 39.” (2022, August 24.) npr.org
Chinen, Nate. “Joey DeFrancesco, driving force on the Hammond organ, dies at 51.” (2022, August 26.) npr.org
“Mountain bike rider Rab Wardell dies aged 37, days after winning Scottish title.” (2022, August 24.) theguardian.com
See “Per4mance Art.”
Thacker, Paul. “Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial.” (2021, November 2.) The BMJ.
Previously reviewed / summarized in “Perfunctory².”
The best rebuttal to the committee’s demonization of the Trump circle’s efforts to redeem HCQ remains Doidge’s critique:
Doidge, Norman. “Hydroxychloroquine: A Morality Tale.” (2020, August 13.) Tablet.
Support for the 60-day trope here https://www.chop.edu/news/long-term-side-effects-covid-19-vaccine








FDA wanted a 60-day review to approve covid vax right after election... but now they tested the Ba.5 vaccine booster on 8 mice and that's "enough according to Peter Marks". Bought 171 million doses of what was not even approved yet!
At any moment after the mail man delivered enough ballots to steal the election for President Poopy Pants the FDA could have said hold-on the ARR is <1% and there are safety concerns. Lets take a step back and review. Lets see what Brooke Jackson is talking about.