The Extinct SARS-CoV-2's in Deer Study
Alpha and Gamma variants detected in deer months after going "extinct" in human transmission.
A new pre-print helpfully shines light on SARS-CoV-2 transmission in an animal population, demonstrating that variants no longer apparent in human infection might persist in deer, at least for several months. Does this mean that the Omicron siblings came from wild animals (no), or that Delta might not be dead after all (yes)?
A minor note: Substack restored a pre-publish draft of this post a few hours after posting. Hopefully the current, correct version stays online this time.
Golden Oldies
The paper:1
The punchline:
These findings indicate that [white-tailed deer] – the most abundant large mammal in North America – may serve as a reservoir for variant SARS-CoV-2 strains that no longer circulate in the human population.
That sounds… great…
If you anticipate deriving celestial enlightenment and subsequent detachment from all material concerns because of this post, please drop a tip now.
Background
Previous findings in a population of deer, in Ohio, showed Alpha circulating in late winter and spring of 2021.2 So: Not the same as finding extinct variants in autumn of the same year, as this new paper does.
The Set-up
A total of 5,462 retropharyngeal lymph node (RPLN) samples collected from free-ranging hunter-harvested [white-tailed deer] during the hunting seasons of 2020 […] and 2021 […] were tested by SARS-CoV-2 real-time RT-PCR. SARS-CoV-2 RNA was detected in 17 samples (0.6%) from Season 1 and in 583 (21.1%) samples from Season 2.
The disparity in positivity rates might seem to be driven by background case rates in New York, where the “Wuhan” model was still “burned out” in 2020 until the winter. In summer and autumn of 2021, the Northeast, including New York, had more human cases:
The Findings
On the other hand, human case rates in the summer might not be principally relevant compared to case rates in late spring, since it seems like crossovers from that era were still transmitting in deer. This is in stark contrast to 2020, which featured an earlier end to the spring wave in New York, and resulted in very few positive PCRs in hunting season:

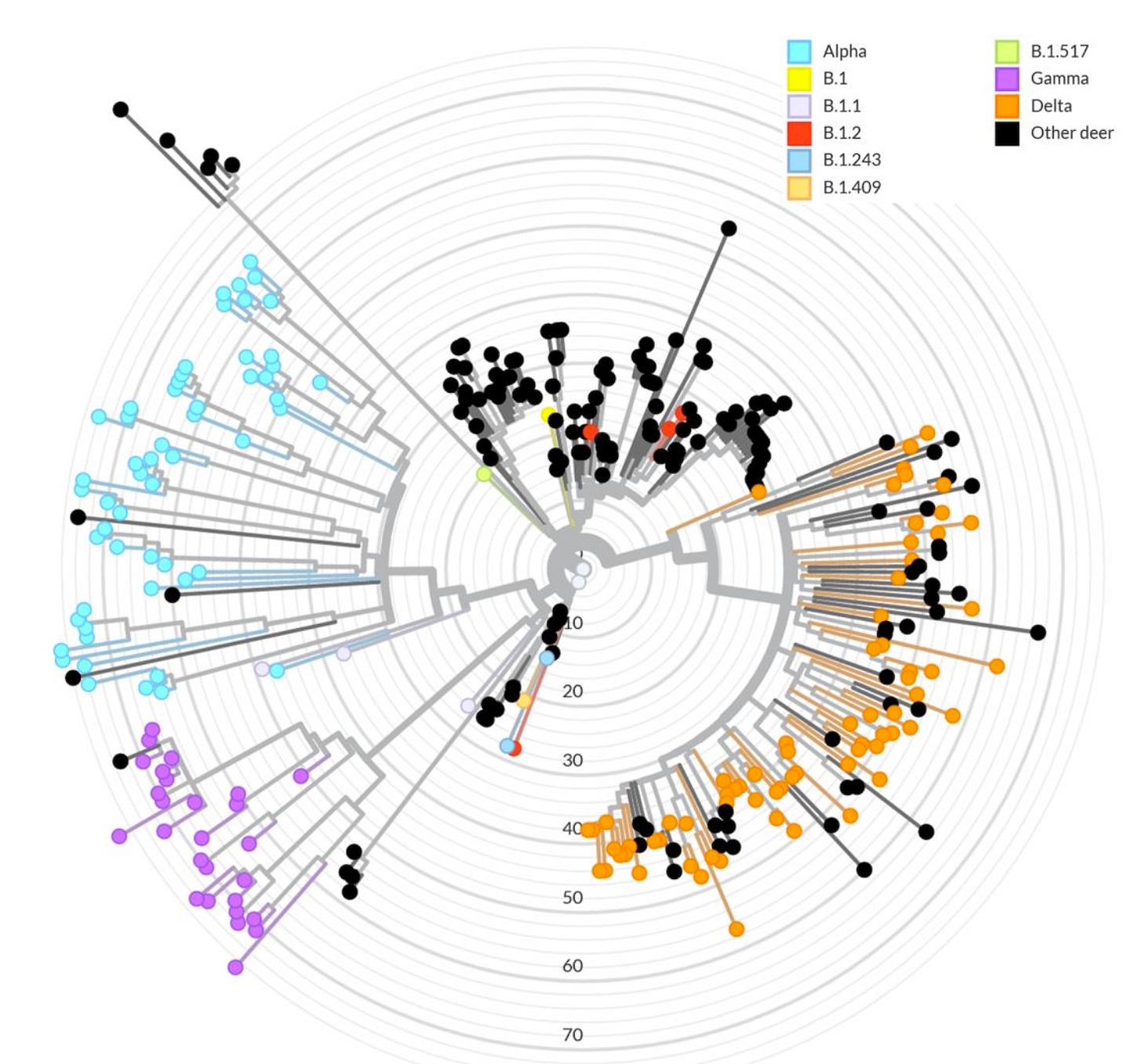
Naturally, the collection of 69 Delta sequences between September and December, 2021, is not surprising — it is consistent with fresh human-to-deer transmission of a virus that white-tailed deer are known to be susceptible to. To collect 27 Gamma and 54 Alpha sequences in the same time-frame, however, suggests that these vanquished human variants were still circulating in this animal reservoir:

Sequences also appear to show an accelerated rate of mutation in deer — not surprising as the virus would be expected to adapt to its new host. Here, I have added “eye-ball-software”-based mutation rates that correct for the authors’ software’s non-assumption of multiple cross-overs:

The difficulty in trying to judge mutation rates this way is that multiple, sequential cross-overs from humans — where the viral genome is more static — will cause the rate of mutations in deer to appear lower than it really is. And so for gamma, presumably the least recently-connected to any human cross-over, the rate appears highest (however, this might be an illusion caused by coincidentally finding the most mutated samples after the least, if transmission in deer had indeed been going on since spring).
For both Alpha and Gamma, at any rate, 2021 season deer had some impressively-mutated sequences, the kind that WHO declarations of “concern” are made of, though not out of proportion to previous sequences from North American deer (see Fig. 5 “wheel” below).
“Footprint” vs. Active Transmission?
Contra the authors, SARS-CoV-2 RNA positivity in lymph nodes might not correlate with active infection or transmission. The expected “baseline” duration of RNA viruses in either human or animal lymph nodes is surprisingly poorly-studied. As discussed in “The 60 Day RNA Mystery,” it may be the case that entire viral particles are frequently preserved in germinal centers for long periods after acute infection.
So, it could be the case that by using lymph nodes as the source material, the authors are revealing a “reservoir” that is even less poorly illuminated than they thought — and one that might not correspond to transmissible virus.
Notably, of 216 samples that were PCR positive at less than 30 cycles, only 7 were successfully cell-culture-recovered in Vero-E6 TMPRRSS2 cells; and the corresponding sequences were all for Delta variants.3

So, perhaps the reason spring-era variants (and even a few vintage B.1’s from before spring) were detected, is that RNA from spring-era deer infections was still lingering in lymph node germinal centers?
Arguing against this interpretation, however, is that the spring variants were detected in geographic and temporally related clusters. For example, sub-30-CT positives which were later sequenced as Alpha and Gamma appeared to “disperse” actively throughout nearby deer, based on collection times — suggesting active transmission, rather than the “footprint” of a spring wave.

While it’s not clear whether or how the authors ruled out that human hunting and collection patterns were actually driving these trends, it’s at least tentative evidence in the “transmission” column (also, sequences appeared truly “sequential,” per the text, revealing “an intricate dispersal pathway with clear connections between the sequences”).
Further, Gamma would be expected to appear more frequently outside of the cluster if long-term retention of non-transmitting viral RNA was common; it is limited to the Allegany area.4
Lastly, extreme mutational distance from any recorded human sequences, and narrowness of ancestry (monophylogeny relative to human sequences), is highly consistent with a single spring human-to-deer crossover that then spent a long time bouncing back and forth in deer.
As the authors put it,
All Gamma WTD sequences detected here formed a monophyletic group highly divergent from the human derived Gamma lineage sequences (Fig. 5A and C).
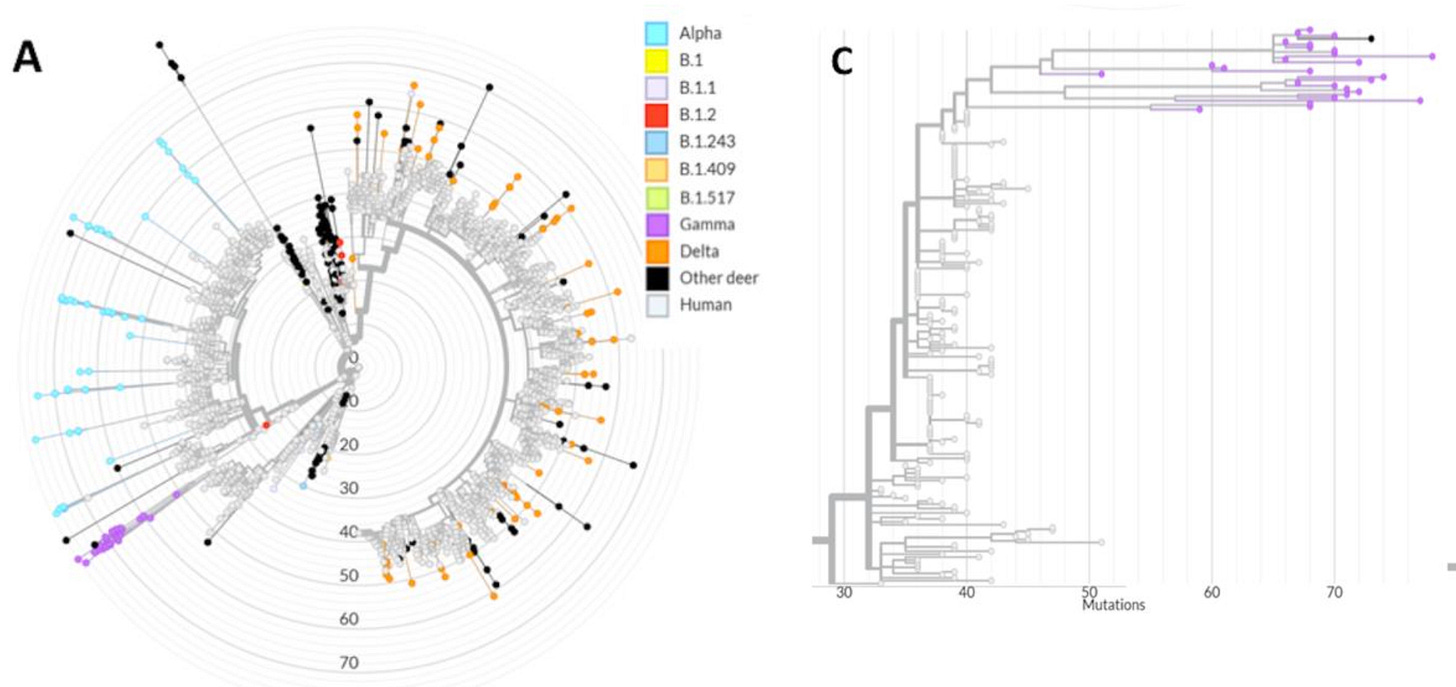
In fact, such a genetic signature would argue that long-term transmission in a zoonotic reservoir, something on the order of a half-year at least, was necessary to produce the sequences collected. So, count me on team “long term transmission reservoir” for now.5
Implications for Past and Future
Does Caserta, et al. vindicate the “Zoonotic Omicron Origin” theory offered to the press by experts last year? Yes and no. It is certainly a proof-of-concept that an early B.1, from spring 2020, could have transmitted for an extended time in wild animals, featuring an elevated mutation rate for some or all of that period. In fact, 4 vintage-B.1-derived genomes are found among the 154 sequences that pass quality check in this study’s autumn, 2021 samples — though they only have on the order of 20 to 50 mutations from the Wuhan reference.6
However, the problem of the simultaneous discovery of the B.1 and B.2 “siblings” still remains; and suggests a lab origin as the most likely explanation for how two such distantly-related strains, with apparent divergent evolutionary pressures, would emerge in the same location.
On the other hand, does this mean that not-so-mild Delta will one day soon be the subject of “I thought you were dead / It didn’t take” memes the world over?
In light of the analysis of the gamma cluster above, I think it is at least demonstrably possible.7
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Hale, VL. et al. (2021.) “SARS-CoV-2 infection in free-ranging white-tailed deer.” Nature. 2022 Feb;602(7897):481-486.
This paper uses nasal swabs instead of lymph nodes.
This could also simply be due to the impossibility of physically separating virus-containing portions of lymph nodes from the immune cells going about their work. That viruses were in germinal centers at all, per my devil’s-advocate theory, is pure assumption. They may have been anywhere, such as the subcapsular sinus or in antigen presenting cells, due to fresh delivery from infection zones. This is in contrast to trying to culture virus from tissues where it is truly actively infectious.
Supplementary table 8 reports two non-cluster Gamma sequences, one of which is in Allegany and the other in next-door Steuben county.
One alternate possibility is a blend of lab-leak followed by in-deer transmission. As discussed in footnote 7, it is certainly likely that human labs will continue to play with old SARS-CoV-2 variants for some time to come. This could lead not just to reemergence in humans, but in animals with shorter lifespans and hence less immune memory. Such a model allows for a delayed introduction of gamma into the “cluster” population. However, once again, the extreme mutational distance from any known human sequences suggests that sustained in-deer transmission did occur.
From Fig. 4. However, it is difficult to tell which of the B.1 sequences are from 2021 and which from 2020; but none exceed 50 (the lavender dots adjacent to the alpha tree).
Note: Reemergence of Delta from the negaverse was predicted here in January.
Of course, it’s arguably just as likely that Delta or another pre-Omicron variant leaks from a lab studying it (by accident this time). So, the determinative factor might be whether humanity has built up enough immune debt for the original generation of the virus. For H1N1 flu, which was kept alive in labs around the world after its disappearance in 1957, lab escape back into human circulation took 20 years. See “1918, I Love You.”
Since circulation in deer adds the element of active mutation, however, it might be expected to more quickly produce a (true) zoonotic re-emergence.











Oh deer! Is this study something the CDC will fawn over? Or will the buck stop here?
......I'll see myself out now.
As someone who grew up in farmer area, but now lives in a deep forest and has been hiking and hunting (pictures only though) in remote areas from childhood, I was fascinated by this deer reservoir first I heard of it over a year ago. I was as it didn't make sense.
First these deer are not herd deer, unlike Elk. The females form sometimes small groups of 2 or 3, but never large packs. They are also fairy stationary. I've had the same female having young in my yard for three years in a row now. In our neighborhood we hence even have names for some of the deer with expressive features, that is how stationary they are.
So it is unlikely this is deer to deer transmission driven. Also the idea that I as a hunter or photographer would infect a deer is ludicrous. I've seen suggestions that they transmit through their own poop or nose-rubbing, but the quantity of infected deer seem to be too high.
(The fact that the researchers never thought this was strange shows these are city folk. :-) )
The most plausible explanation I heard discussed is poop spraying. Unknown to most people is that many American states use wastewater to spray lands. This spraying goes on for hours and creates large clouds of mist. Also realize that unlike the mountain states (Montana etc) and the West Coast most states don't have true vast virgin forests but large mixed use areas where agriculture and forested areas meet. Deer hence frequently roam around farmland getting exposed to these poop-mists. Time of deer activity and time of spraying also focusses around evenings.
So my guess is that these are not primarily deer-to-deer, but just poop sprayed infected. Various studies showed that they could easily not just detect but easily get live samples from wastewater.
Then indeed as pointed out, the virus remains likely just in the lymph node for a long time.
Of course I could be wrong, and there is truly an animal reservoir. That would be very troublesum, but my bet is it is just that - poop spraying.