Pfizer Did Not Know What the Covid Vaccines Actually Do in the Body
The IgG4 debacle in light of the gaps in the supposed "development" of BNT162b2.
What Goes on After the mRNA Vaccine Is Injected?
Two years after the Emergency Authorizations that triggered widespread deployment, we’ve learned very little. Perhaps even more galling, the question was never investigated in the original trials.
This post:
Reviews how “pharmacology” of the Pfizer/BioNTech vaccine was never directly assessed during either the development or perfunctory “trials.”
Blotter-gate: Pfizer simulated proof that truncated RNAs don’t get turned into proteins?
Materials, as already uploaded by others:
Described by Sasha Latypova as “a document titled “COVID-19 Vaccine (BNT162, PF-07302048) BB-IND 19736 S.2.2 Description of Mfg Process and Process Controls (modRNA) [BNT Mainz and Rentschler]”, submitted to the FDA.”1 December, 2020.
Described as “Pfizer’s Chemistry Manufacturing and Controls (CMC) documentation submitted to the European Medicines Agency (EMA) and Therapeutic Goods Administration (TGA) in Australia.” August, 2021.
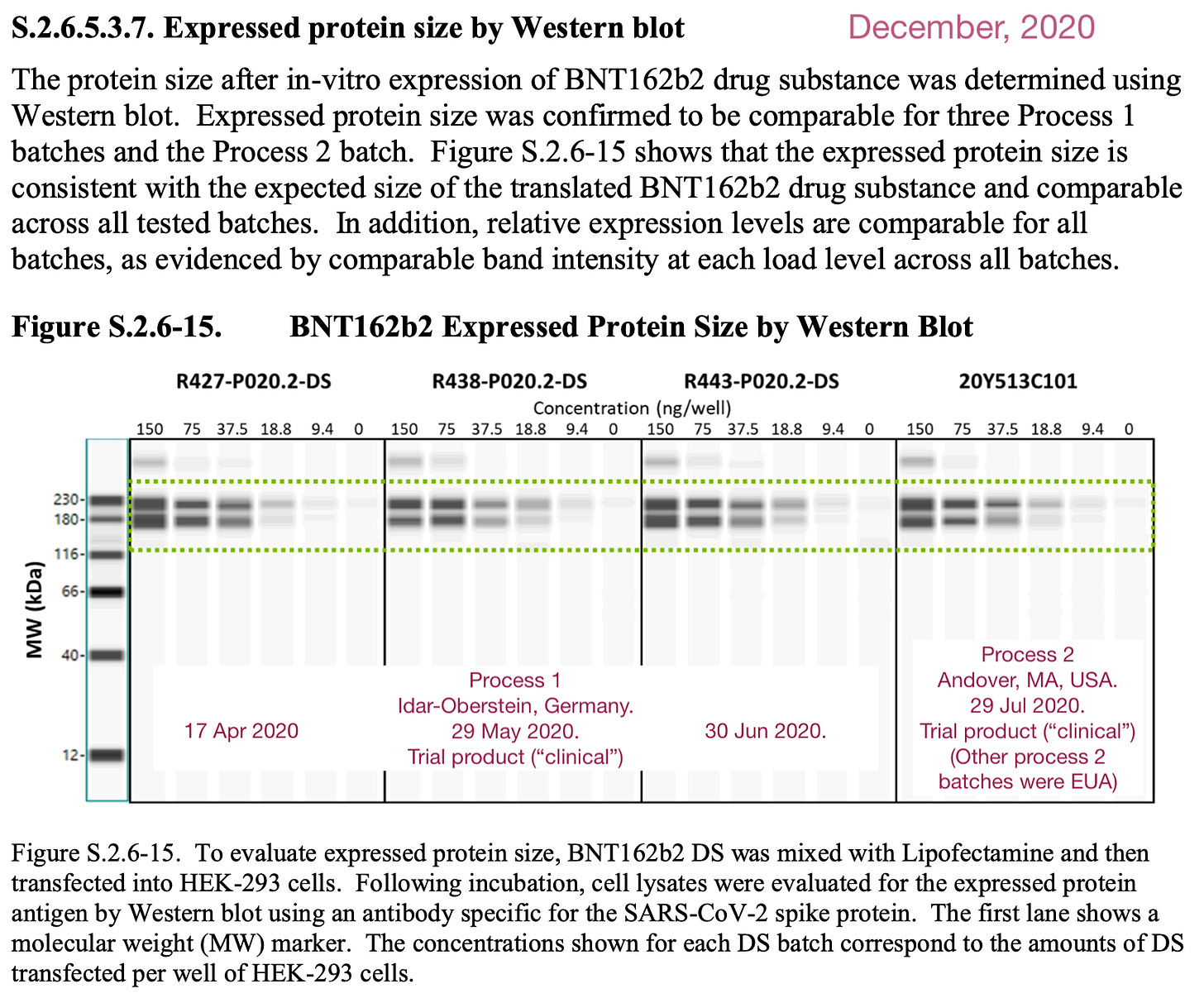
i. Did Pfizer / BioNTech’s product make full-length spike?
Recently, numerous writers have highlighted the discovery, initially reported by “Jikkyleaks” on twitter, of blatantly computer-simulated Western blot images in materials submitted by Pfizer to multiple perfunctory “regulators.”
Essentially, these agencies tasked Pfizer/BioNTech, inter alia, with the seemingly trivial job of showing that the product they were claiming “causes cells to produce the SARS-CoV-2 spike protein”… causes cells to produce the SARS-CoV-2 spike protein. Surely not beyond the powers of two billion-dollar corporations combined.
In December, just before deployment, Pfizer sent the FDA Western blot “images” of Covid-vaccine-expressed spike protein that were clearly not images of Western blots, but either computer simulations or “touch-ups.” From the Manufacturing Process Development report:
It should be noted that virtually everything in the Manufacturing Process Development report looks either computer-processed or computer-simulated, except for the RNA gels (further below).
Processed, noise-free images might pass for chromatograms, if Pfizer’s practice was simply to make results legible for regulators that wouldn’t necessarily have used the tools in question, and if raw results could be provided on request (both these pdfs are more reader-friendly than the raw experiment results included in the Nonclinical and Clinical Overviews, for example).
But Western blots are subject to strict research taboos in terms of showing valid images, which will have a distinct “melting” character and generally pack in circumstantial markers that can help third parties detect fraud2 — markers which are erased by computer touch-up, if that is what took place here.
For this reason, tools to computer-process Western blots have no function and shouldn’t even exist. Instead, per a comment from one of Latypova’s associates, there are tools that generate exactly the clearly-fake “demo results” that Pfizer submitted.
However, the program used to generate those fake blots is clearly not optimized for creating a fully true-to-life impression. I think that the programmers did it this way deliberately -- they wanted to discourage people from passing off this stuff as real.3
Edit, January 17:
SimpleWestern’s automated blot machines do, in fact, directly convert from "real" blot to synthetic blot based on detected protein levels.4 In a paper coincidently (?) published on January 12, Pfizer self-reports having used SimpleWestern for the truncated protein experiments in the next section. As matches my originally-published interpretation of the assessor’s remarks, regulators were likely already aware of this processing step and may have had access to perserved, original blot images (including lanes not represented in the mock-ups).
Was it simply Pfizer’s practice to process noisy readings in order to make them digestible to regulators who might be less literate in reading raw results? Or was it instead the case that Pfizer and BioNTech did not actually have possession of the product that was being injected into trial participants?
ii. Falsifying truncated protein results?
Rationale for worrying about truncated proteins.
Regulators also asked Pfizer and BioNTech to demonstrate that besides making full-length spike protein, their product did not induce cells to make truncated, or partial proteins of who-knows-what composition and biological character. This request was not met in time for widespread deployment.
BioNTech finally submitted results in August, 2021, shortly before mandate-enabling “approvals” began to be rubber-stamped onto the product that had already been injected into 10s of millions.
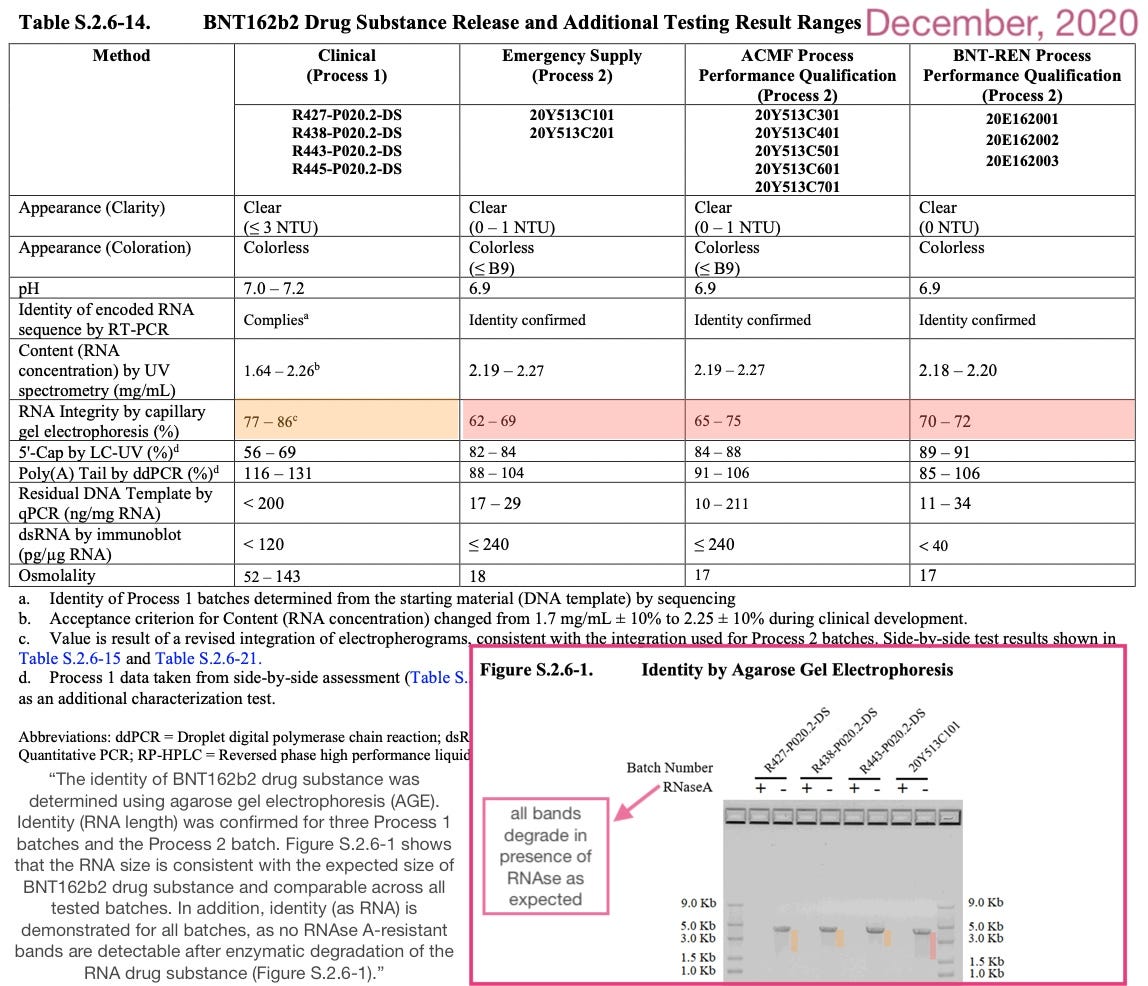
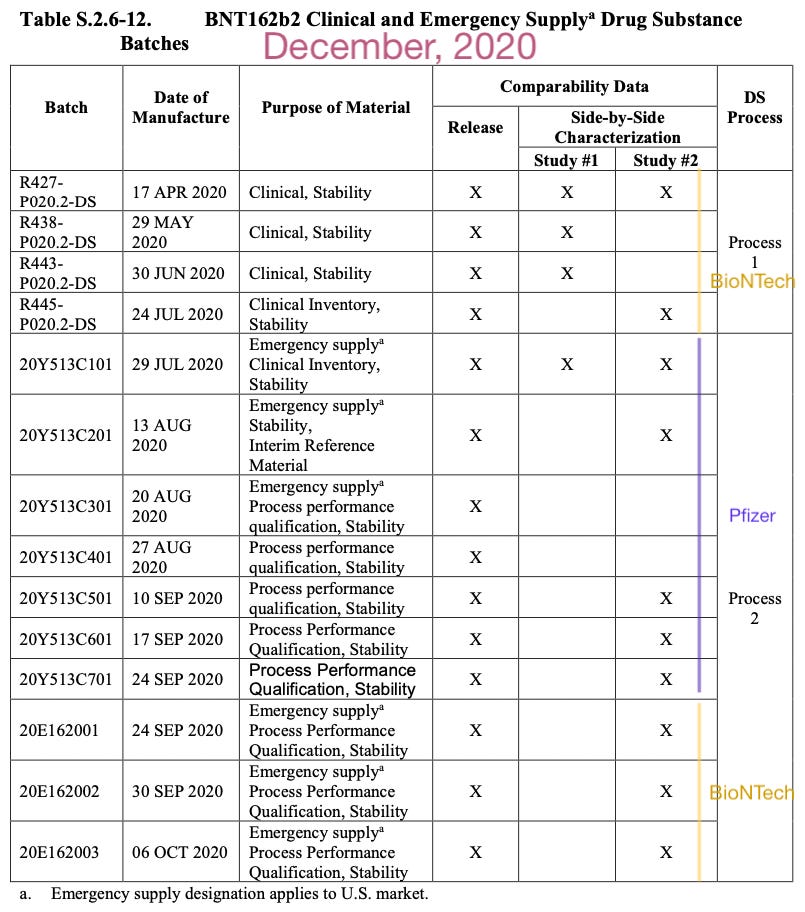
The regulators’ concern arose because it was known that even in the first 14 in-house batches made by BioNTech and then Pfizer, substantial truncated (as in, fragment) RNA molecules were mixed in with the desired full molecule.
RNA is fragile, so even if both firms could produce 100% perfect full-length spike-encoding RNA molecules, lots of them get broken apart when they are processed, packaged, etc. There was nothing the regulators could do in December, 2020 but accept this fact.
Note the RNA agarose gel electrophoresis results are “authentic” in so far as representing the product of an actual experiment. Of course, nothing prevents these corporations from ordering some other RNA molecule of the expected length on Amazon and shooting it through some gels. Nonetheless, it is at least consistent with their having actually had prototype Covid vaccine on hand to test (as would be consistent with their actually producing the same).
With such high percentages of fragment RNA, the European Medicines Agency in particular wanted to know that these fragment molecules don’t get turned into fragment proteins. (You might ask why wouldn’t they? In theory, incomplete molecules would, either, lack the 5’ “cap” required for ribosomes to process them into proteins, or be instantly degraded by exosomes once inside cells, due to lack of either the cap (again) or the “don’t degrade me, bro” poly-A tail required to keep mRNA viable. Without both cap and tail, RNA molecules cannot be a “messenger” inside the cell.)
It should also be noted that by this point, most people would have been receiving derivative, “scaled up” versions of the prototype Process 1 and 2 batches, which might have borne no resemblance to whatever quality benchmarks were achieved by those early prototypes. This has also been extensively discussed by Latypova and Maria Gutschi.5 Essentially the EMA's truncated protein “Special Obligation” (SO2) was totally perfunctory at this point. “Using the best versions of your product, which weren’t even deployed in the emergency authorization,6 can you at least pretend to show that it doesn't make a bunch of mutant proteins?”
BioNTech’s response.
BioNTech devised and submitted the results of three experiments to meet this perfunctory requirement:
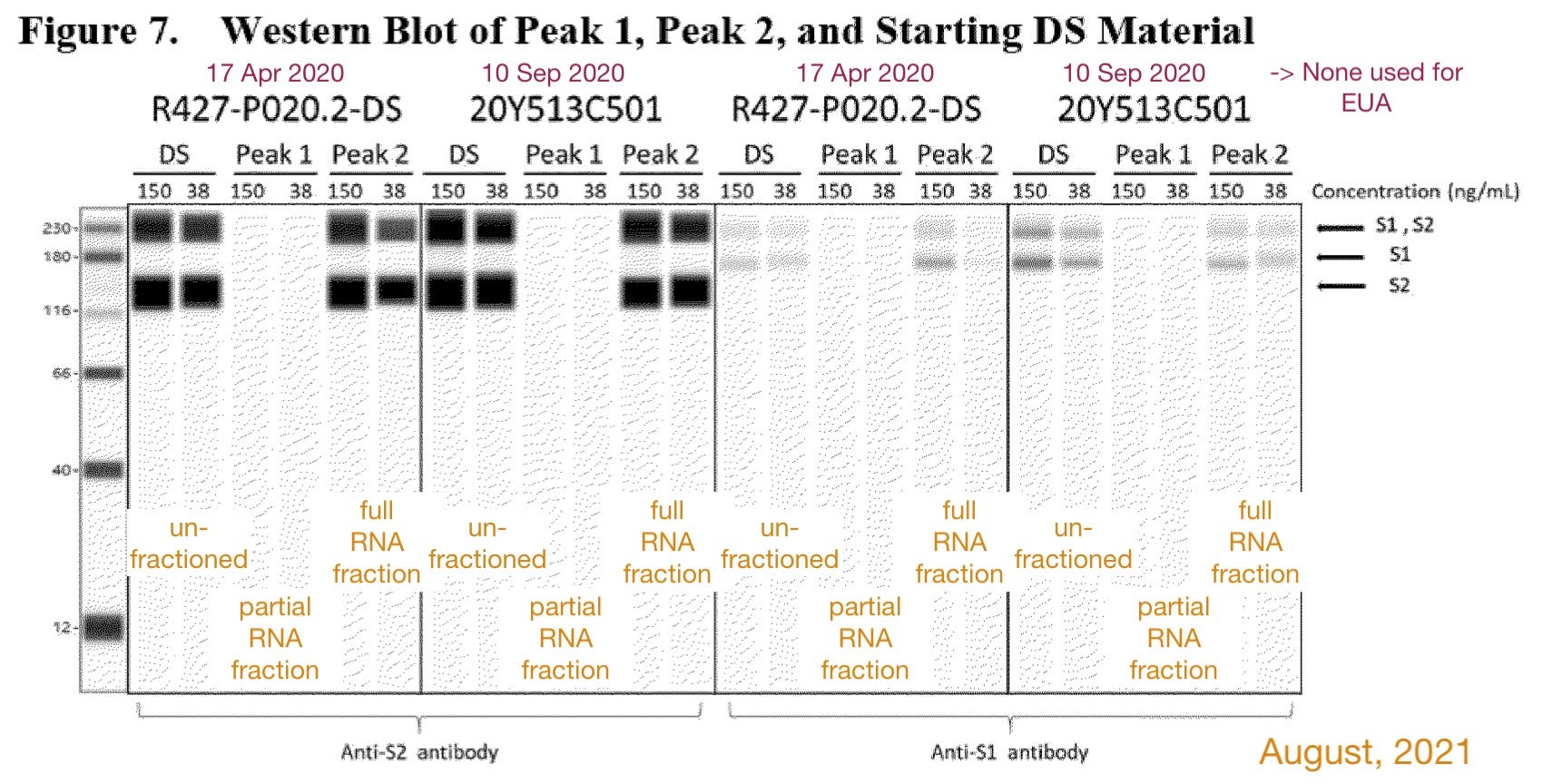
BNT162b2, their Covid vaccine product, would be fractioned to divide the partial (“Peak 1”) and full (“Peak 2”) RNAs. It would then be shown that partial RNAs don’t generate proteins in vitro. [Cue fake blot.]
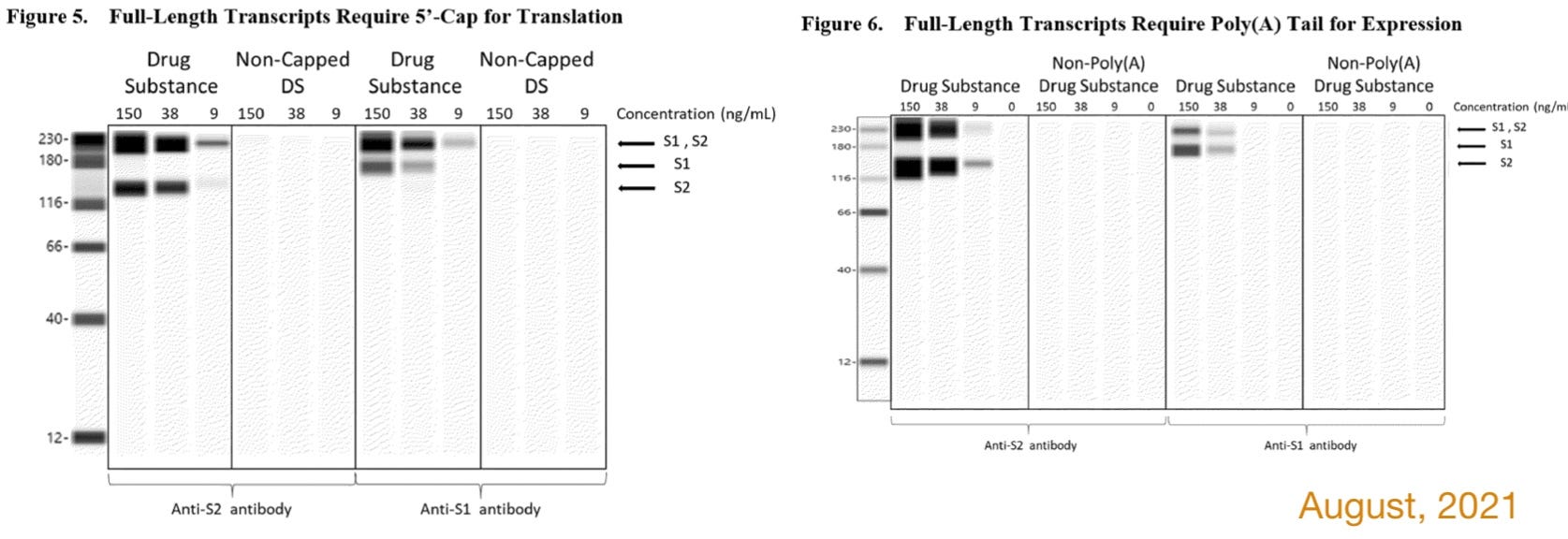
It would be shown that full-length BNT162b2 RNAs lacking in the “cap” or tail do not produce spike protein in vitro. [Cue fake blot.]
It would be shown that BNT162b2 can be intentionally degraded to “amp up” RNA fragments without the appearance of novel protein products. For this, rather than another transfection of cells, BioNTech used a “busted open cell” system in which any protein should show up due to indiscriminate inclusion of labeled “K” residues.

Suddenly: A real blot?!
iii. Afterward: The assessor’s remarks.
As others have commented, the lack of objection of the EMA and other regulators to these multiple, obvious simulated blots is astonishing. The “assessor” in the assessment report is clearly familiar with the methods being assessed (or, had been provided a canned script in a separate communication) (emphasis added):
To assess the potential for translation of mRNA fragments into truncated S1S2 proteins/peptides or other proteins/peptides, two orthogonal protein expression systems were utilized and translated proteins were evaluated by Western blot. To test the hypothesis that transcripts require both 5’-cap and poly(A) to support protein translation, full-length transcripts lacking either 5’-cap or poly(A) tail were transfected into HEK-293 cells, and the resulting cell lysates were analysed by Western blot using detection antibodies specific for the S1 or S2 domain. The WB indicates that transcripts require both 5’-cap and poly(A) to accomplish protein translation, since no protein bands can be observed for the truncated species [on these clearly synthesized images!]. However, WB is an epitope specific method, and truncated variants of the proteins [that would not light up with spike antibody] could potentially be generated. Nevertheless, in line with the results obtained on full-length transcripts, cells transfected with the starting DS batches (R427-P020.2-DS and 20Y513C501) or with Peak 2 material (isolated using IP-RP-HPLC above) show comparable spike protein expression by Western blot, whereas cells transfected with Peak 1 material, comprised of fragmented species, do not show evidence of protein expression [on these clearly synthesized images!]
[…] SO2b is considered solved.
Incredibly, the assessor then goes on to dismiss the cell-free system — the only authentic Western blot was not accepted in the rationale for solving SO2b!
However, no details are provided regarding the experimental setting for this characterization exercise which impairs evaluation of the data provided. The method used should be appropriately described, including conditions for in vitro reaction, antibodies used and choice of investigated material. Also, additional BNT162b2 batches with different levels of RNA integrity at release are expected to be included in the characterization exercise.
What makes this decision odd is that the documents in question are not exactly verbose in describing the experimental conditions for every other module. Given what is included in these leaked documents, any of the experiments could have been criticized for some degree of ambiguity in “describing conditions.”
For this reason, I would propose that the assessor was pre-familiar with the other Western blot experiments which are represented by synthetic images, but not with the experiment represented by an authentic image.
In light of this quirk in the assessor’s remarks, it is entirely possible that multiple authentic Western blots had been previously shared by BioNTech in other communications, and the synthetic images were merely meant to “display” the derived results.
Or it could be that neither Pfizer nor BioNTech had access to the actual “Covid vaccine” product labeled with their brands at any point.
I’m sure it will all be cleared up some time before the year 2300.
iv. Afterward 2: Patel, et al.
Pfizers turns out to have self-published these damning images a week after jikkyleak’s original tweet. Comments in footnotes.7
Pfizer openly states that true “Pharmacology” was never assessed.
Oxford Languages defines “pharmacology,” ahem, as “the branch of medicine concerned with the uses, effects, and modes of action of drugs.”
The language in the Pfizer nonclinical report clearly throws that definition out the window: Covid vaccine “pharmacology” was defined as immunogenic responses in both animals and human Phase 1 and 2 trial participants. Thus pharmacology — what actually goes on in the body when these products are injected — was, we are to believe, never truly assessed.
Essentially, Pfizer and BioNTech collectively purport to have tested how their Covid vaccine works in actual, living creatures simply by measuring antibodies and other markers. One could liken this to a car company selling you a car for which all the “testing” amounted to seeing whether people showed up somewhere. They didn’t look at the actual driving of the car.
Pfizer and BioNTech thus could not actually know that their mRNA transfections would lead to such durable expression of spike protein that IgG4-mediated tolerance already emerges after the primary course, because they never looked into expression kinetics over time to begin with.
This is no great, big secret — but thanks to a prompt from the blogger who goes by Sage Hana, I was recently reminded that it is actually in practice a great, big secret. It hides in the labyrinth of disclosed details in the Pfizer clinical overview8 and the nonclinical report submitted to the Australia's TGA and other regulators.9
From the former:
Key nonclinical evaluations of BNT162b2 included pharmacology (mouse immunogenicity studies, non-human primate [NHP] immunogenicity and challenge studies)"

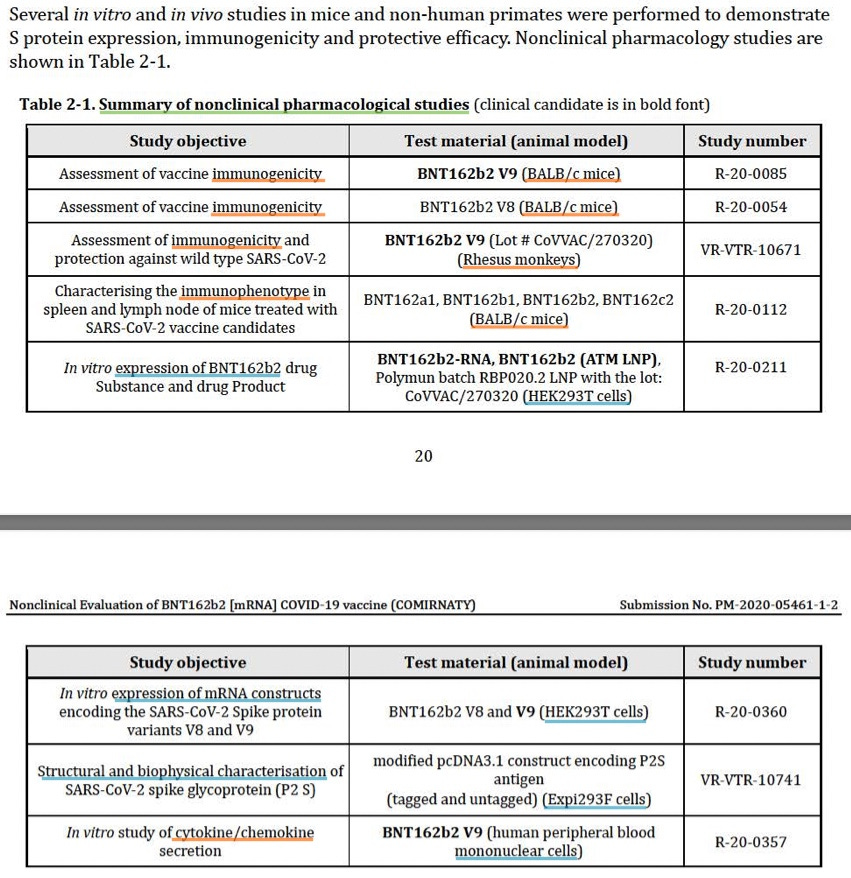
“Pharmacology” = immunogenicity. These experiments are summarized and detailed starting on page 20 of the nonclinical report, so it is easy to verify that the language above is not over-simplifying one iota.10
Continuing from animals to humans, the experiments begin in Phase 1: Immunogenicity, ie antibodies and T Cells, were used to pick between the two remaining candidate RNA models. There was at no point any testing on what was actually happening between injection and said antibodies and T Cells.
BioNTech has evaluated multiple RNA-LNP platforms, including nucleoside-modified RNA (modRNA) which has blunted immune sensor activating capacity and thus augmented antigen expression [in theory; they just measured antibodies in mice and saw a small increase in modRNA vs. not]. Two modRNA candidates were evaluated in the Phase 1 portions of studies BNT162-01 and C4591001. The final candidate and dose level (BNT162b2 at 30 μg) was selected following review of the immunogenicity and safety data from the Phase 1 part of study C4591001 and available nonclinical data.

Pfizer and BioNTech allegedly ruled out all possible variations of RNA construct besides two by simply trying out four versions on a few animals to see how many antibodies and stuff they made.

This was followed by injecting a handful of humans with b1 and b2, and measuring… antibodies and T Cells.
And after this staggering, Manhattan-Project-like effort, they just “coincidentally” picked the same design strategy as Moderna — modRNA for proline-stabilized full spike.
At no point was true pharmacology of BNT162b2 ever assessed. The famous “rat distribution” study comes closest. It at least uses the same LNP construct as BNT162b2, but with RNA encoding luciferase rather than the spike protein. As openly admitted in the introduction:
At no point did Pfizer or BioNTech have any idea what their product was doing or would actually do in humans.
Which naturally leads one to imagine whether someone else — i.e., BNT162b2’s actual creator, if it was not these two companies; or the folks at Moderna whose design-decisions BioNTech emulated — did.
Related:
If you derived value from this post, please drop a few coins in your fact-barista’s tip jar.
Latypova, Sasha. “Fake Western Blots Submitted by Pfizer to Several Regulatory Agencies.” (2023, January 10.) Due Diligence and Art.
Piller, Charles. “Blots on a Field?” (2022, July 21.) Science.
(Latypova, Sasha.)
Latypova, Sasha. “Maria Gutschi, PharmD, on Lack of Manufacturing Quality of mRNA Injections.” (2023, January 3.) Due Diligence and Art.
Patel, HK. et al. “Characterization of BNT162b2 mRNA to Evaluate Risk of Off-Target Antigen Translation.” Journal of Pharmaceutical Sciences.
Supports my interpretation of the regulators being pre-aware of the processing step. Patel, et al. casually report that for the experiments besides the cell-free degradation assay, “mRNA sample results are reported as ProteinSimple Wes assay run lane images.”
Contra my original text, ProteinSimple does constitute a tool to convert real blots directly to touched-up, simulated blots. I.e., the tool that I reported should not exist, exists (it is always helpful when brand names are specified). I have edited the text of this post with the original version preserved in strikethrough.
Reveals that Pfizer has either changed its mind or never was in agreement with the theory (prevailing in the reports submitted to regulators) that most partial RNAs were the product of degraded full RNAs. Patel, et al. now opine that most partial RNAs are incomplete transcriptions. This conclusion is portrayed as stemming from the experiments in question, as if to imply that no further work has been done to investigate manufacturing quality besides these few experiments on pre-EUA batches:
The data demonstrate that BNT162b2 fragments originate predominantly from premature transcriptional stops during manufacturing and do not have the capacity for protein translation, as evidenced by orthogonal protein expression systems.










HOW DID I NOT SEE “BLOTTER-GATE” AT FIRST
Corrected.
I bet someone knew what this garbage would do in people's bodies, but certainly not Pfizer execs: https://odysee.com/@slikrik2003:0/pfizer's-dr.-bill-gruber-doesn't-know:a